SuperNHS
Data Sheet
![]() Shop this product in our online store
Shop this product in our online store
Products - Microarray Substrates & Slides - SuperNHS Microarray Substrate Slides with Highly Reactive NHS Esters for DNA, Protein and Small Molecule Microarrays

Arrayit NHS-Ester Microarray Substrate Slides provide the market's finest surfaces for whole cell, protein, carbohydrate and small molecule microarray printing and experimentation. Based on highly advanced tri-functional organic chemistry and semi-conductor quality class 1 cleanroom manufacturing, Arrayit's new SuperNHS Slides offer high loading capacity and extremely low background when ultimate signal-to-noise ratios are essential. Our atomically smooth glass substrate slides are reacted with the first portion of the tri-functional polymer to produce a highly durable coating resistant to the broad range of temperatures and reactions conditions used in microarray assays. The second portion of the polymer contains highly reactive n-hydroxysuccinimide (NHS) ester groups, which form avid covalent bonds with primary amines and other nucleophiles present on the surface of whole cells, proteins, carbohydrates, small molecules and other printed sample molecules. The third polymer moiety contains a hydrophilic functionality that shields the glass surface from non-specific binding to produce remarkably low background. This two-dimensional, highly transparent surface chemistry is highly recommended for whole cell assays, proteomics, glycomics, high-throughput drug screening, and all other applications requiring a premium microarray surface.
Table of Contents
- Introduction
- Quality Control
- Product Description
- Technical Note
- Technical Assistance
- Short Protocols
- Complete Protocols
- Recommended Equipment
- Troubleshooting Tips
- Ordering Information
- Storage Conditions
- Warranty
Introduction
Congratulations on taking a big step towards improving the economies of scale, quality and speed of your proteomics research. This booklet contains a complete set of protocols outlining the steps and principles needed to use ArrayIt® SuperNHS Substrates.
Quality Control
Arrayit assures the performance of this product. The finest scientific research went into the development of this product. Rigorous quality control monitoring on a piece by piece basis guarantees that the product exceeds the highest industry standards.
Product Description
ArrayIt® SuperNHS Microarray Substrates provide the highest quality glass microarray printing substrate at an affordable price. All of our substrates are manufactured in state-of-the art class 10 clean rooms, with 0.1 µm filtered air, and temperature and humidity control. Cleanroom manufacturing eliminates contamination of the microarray surface with particulates, proteases, nucleases and other contaminants that impair the quality of microarray experimentation. Compare our SuperNHS Substrates to traditional microarray slides and you will see the difference.
Users will appreciate the following product features:
- Advanced tri-functional polymer activates the glass surface
- Polymer provides a highly durable coating
- Highly reactive n-hydroxysuccinimide (NHS) ester reactive groups
- Hydrophilic third polymer moiety shields the surface from non-specific binding
- Bind whole cells, proteins, carbohydrates and small molecules
- Polished to atomic surface smoothness <±20 angstroms over 1.0 µm2
- Superior substrate flatness of <0.036 mm over 25 x 76 mm
- Only polished glass substrate/slide in the microarray industry
- Homogenous NHS-ester treatment provides superior reactivity
- NHS ester surface density of 3 x 10^12 groups per mm^2
- Vastly superior to unpolished or flame polished glass from other vendors
- Ultimate surface for ultra-high density DNA and protein microarray manufacturing including micro-mirror, photolithography and contact printing
- Topological smoothness ensures uniform hybridization layer and scanning
- Manufactured in a state-of-the-art class 1 cleanrooms
- Ultra-low intrinsic fluorescence and background noise
- Open platform dimensions compatible with all major brands of microarrayers and scanners
- Precise physical dimensions (25 ± 0.2 mm x 76 ± 0.3 mm x 0.940 mm ± 0.025 mm)
- Proprietary corner chamfer (1.4 mm) provides unambiguous side and end orientation to simplify printing and processing
- Finished edges enhance user safety
- Excellent refractive index, transmission and hardness specifications
- Vacuum anti-static packaging improves usability and increases shelf life.
- Offered with or without bar-codes
- Custom laser and chrome fiducials available upon request
- Product arrives “ready to print” with no additional processing required
- High-volume 100,000 piece per month manufacturing capabilities
- Free of particulate, protease and nuclease contamination
- High efficiency covalent coupling via highly reactive NHS-ester reactive groups
- Protein coupling via surface lysine and arginine residues
- Supports both contact printing, ink-jet and hand held printing
- Protein coupling complete within minutes after printing
- No cross-linking or baking required for coupling
- “Zero” background fluorescence
- Uniform NHS-ester density of ±2% across the substrate
- Print proteins, enzymes, antibodies, receptors, antigens, and peptides
- Print pure proteins, recombinant proteins and cellular extracts
- Uniform feature size
- 25 substrates per box
- Anti-static and impact resistant packaging
- Three month shelf life at room temperature
Technical Assistance
Please contact us if you have any comments, suggestions, or if you need technical assistance. By electronic mail: arrayit@arrayit.com (under the subject heading, please type, “Technical assistance”). By telephone: (408) 744-1331, Monday–Friday, PST 9:00am - 4:30pm. Please remember that we want to hear about your successes!
Figure 1. Correct Substrate Orientation. Shown is a graphic of two ArrayIt® Microarray Substrates, showing the correct and incorrect orientation for use. In the correct orientation (blue graphic), the chamfer will be located in the upper right corner and samples should be printed on the side facing upward, which is the same side that contains the word “Correct!”. In the incorrect orientation (red graphic), the chamfer will be located in the upper left corner, placing the backside facing upward, which is the side that contains the word “Incorrect!”. Only one side of ArrayIt® Microarray Substrates is suitable for printing. Please print on the correct side only.
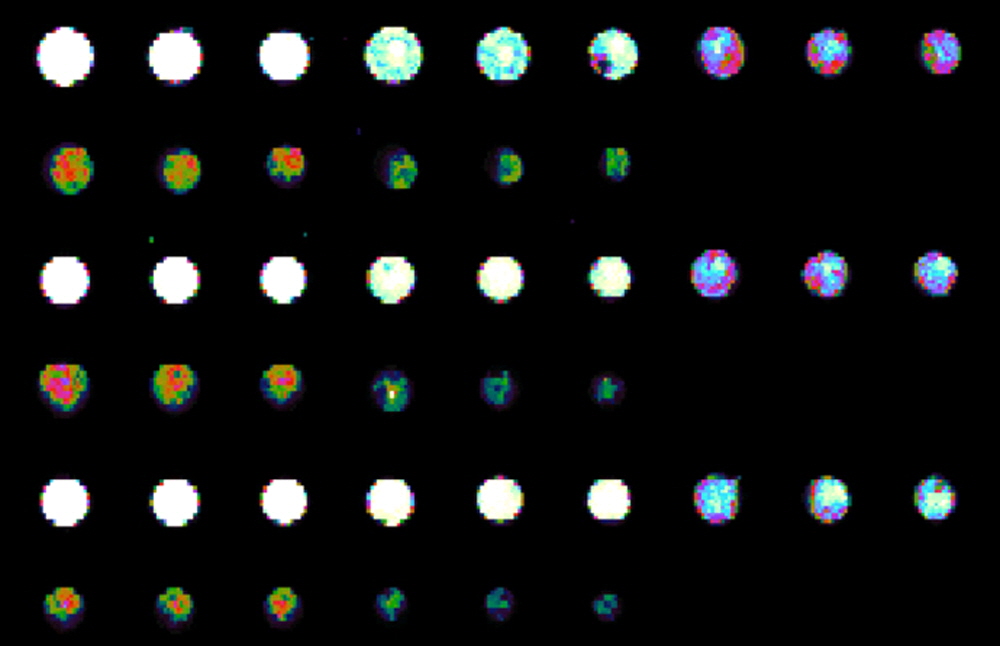
Figure 2. Simple test to determine the best concentration of protein to print onto SuperNHS. Whole Human IgG is printed in Arrayit Protein Printing Buffer using 946MP3 Microarray Printing Pins and a NanoPrint Microarrayer. Three different print runs left to right top down with final concentrations in triplicate at 0.5, 0.25, 0.125, 0.0625, 0.03125 µg/µl final concentration. 16-bit raw data above presented in pseudo color rainbow pallet provided by ImageJ. Microarrays were processed in an AHC4x24 and reacted with 1/1000 dilution of anti-human IgG conjugated to a fluroescent dye and scanned with an Innoscan 710AL Microarray Scanner, gain 1, laser power low, at 10 micron resolution. 0.5 µg/µl concentration protein works best to fully saturate the binding sites as that provided saturated signals at the lowest possible sensitivity settings on the scanner. To download and view the consistant raw data from all 96 microarrays in this test point your browser to: http://www.arrayit.com/Arrayit_Corporation/SuperNHS-data.zip.
Protein Short Protocol (Steps 1-7)
1. Suspend protein samples in Protein Printing Buffer at 0.5 µg/µl.
2. Print protein samples onto SuperNHS Substrates.
3. Block with BlockIt or Blockit Plus and Wash/Rinse the printed protein microarrays.
4. React the processed microarrays with fluorescent samples.
5. Wash the microarrays to remove un-reacted fluorescent material.
6. Scan the microarray to produce a fluorescent image.
7. Quantitate and model the fluorescent data.
Protein Complete Protocol (Steps 1-7)
1. Suspend protein samples in Protein Printing Buffer. Obtain 0.5 µg/µl protein samples in 1X phosphate buffered saline (PBS) and add an equal volume of 2X Protein Printing Buffer. Mix the samples by pipetting up and down 10 times. Protein samples should be free of aggregates and particulates that can clog printing devices and impair attachment to the microarray substrate. Aggregates and particulates can be removed by centrifugation, dialysis and/or filtration. A 50kD protein at 1 µg/µl concentration has a concentration of 20 µM. Certain proteins or protein extracts are more stable at 4°C. Keeping the protein samples cool may improve protein stability while they are being printed into a microarray. Stability can also be improved in some cases by the addition of protease and phosphatase inhibitors, or by the use of a SpotBot Protein Edition Personal Microarrayer or NanoPrint Protein Edition Enterprise Level Microarrayer equipped with a cooled platen or plate cooling apparatus. Make sure protein samples are mixed thoroughly before printing.
2. Print proteins, amino linked glycans or other biomolecules containing sufficient primary amines to covalently bind onto SuperNHS Substrates to form amide linkages. The SuperNHS surface couples proteins extremely efficiently owing to the high reactivity of the NHS-ester. It is good protocol to let microarrays sit overnight prior to processing. Printed microarrays can be stored unprocessed to protect coupled molecules. Protein Printing Buffer contains components that stabilize printed proteins for long term storage. Processing (blocking and washing prior to reaction) is typically performed just prior or within days to use for best performance.
3. Block and process the printed protein microarrays. Prior to using the microarray, wash the printed microarrays to remove unbound protein molecules and buffer components from the SuperNHS Substrates. Protein binding to the SuperNHS surface is extremely stable and the microarrays can be washed, blocked and reacted without sufficient loss of coupled protein. For best results block the surface using 1X Blocking Buffer or Blockit Plus at least 1 hour and as long as 24 hours. Use 4 deg C for long term applications. Functional results can be obtained with a 60-minute incubation at room temperature in other traditional protein based blocking buffers used in ELISA assays and western blots, but the purity of reagent is more important in miniaturized microarray assays. A High Throughput Wash Station or an equivalent device can be used for washes with pre-made protein microarray wash buffers. Blocking can be performed with very gentle buffer agitation and the stir plate set on a low speed or under a coverslip. The blocking step will couple reactants to the open NHS-esters on the surface and prevent background fluorescence. After blocking, wash the microarrays to remove the excess blocking buffer. Washing three times for 2 min each at room temperature with Protein Microarray Wash Buffer.
4. React the processed microarrays. See tools section for helpful links and products. Processed microarrays containing coupled target proteins can be reacted with samples to accomplish a variety of binding assays. Fluorescent, chemiluminescent, colorimetric and other detection techniques are all possible. Binding reactions can be performed in Protein Microarray Reaction Buffer or 1X BlockIt buffer + labeled or non-labeled proteins, cellular extracts or unlabeled protein mixtures such as serum. Optimize dilutions for your specific application, but 1 to 300 dilution of serum in Protein Microarray Reaction Buffer has proven to work well. Fluorescent samples can be incubated as a droplet on the printed microarray, underneath a cover slip, or in a micro-fluidics chamber. A 60-minute incubation at room temperature is usually sufficient to obtain strong binding and intense fluorescent signals (see Fig. 1). Reactions can be performed at 37 degrees C or at room temperature. Use 37C for serum reactions. A Hybridization Cassette can be used to prevent sample evaporation during prolonged (1-12 hour) binding reactions. Multiplexed reaction cassettes are available. Mixing and temperature control can be accomplished with the Array Plate Hybridization Station and Arrayit reaction tools.
5. Wash the microarrays to remove un-reacted material. Once the binding reaction between the bound target proteins and the fluorescent protein probe molecules is complete, wash the microarray to remove the unbound fluorescent material. Washes can be performed three times for 5 min each at room temperature in Protein Microarray Wash Buffers in a High Throughput Wash Station, petridish with agitation or equivalent device. After the wash procedure, excess buffer should be removed from the surface by tapping the substrate on a lint-free cloth or by centrifugation with a Microarray High-Speed Centrifuge.
6. Scan the microarray to produce a image that can be quantified. The fluorescent protein microarray can be scanned or imaging using any of a number of high quality commercial detection instruments including ArrayIt® Innoscan, ArrayPix and many others. Instrument laser/pmt settings should be adjusted to saturate only the strongest signals for a quantitative image.
7. Quantitate and model the fluorescent data using Mapix or other quantification software program. Protein microarray data from the fluorescent image can be quantified, mined and modeled using many different commercial software packages.
Storage
When using SuperNHS it is best to use the product within 6 months of the manufacturing date. Keep clean and dry. For example, lot 110725, was made on July 25, 2011. Once microarrays are printed, samples are covalently bound and stable for long periods of time. Protein microarrays scan be stored at 4C or at room temperature. Microarray storage conditions are clean and dry. Microarrays stored at 4C should be enclosed in environmental proof containers and allowed to come to room temperature before opening. These substrates are made often and customers are always guaranteed a fresh lot. SuperNHS can also be made to order and made on custom size substrates, microfluidic chips and wafers, Contact arrayit@arrayit.com for more information.
Troubleshooting Tips
Poor printing quality:
- Incomplete mixing of protein samples
- Poor printing environment (50% humidity and 25°C recommended).
- Poor sample preparation
- For more information on troubleshooting printing tips please click here.
Poor protein coupling:
- Contaminants in samples
- Poor printing buffer (PPB highly recommended)
Weak fluorescent signals:
- Poor binding of fluorescent proteins
- Probe labeling inefficient
- Washes too harsh
Recommended Equipment and Reagents
NanoPrint™ 2 Microarrayers
SpotBot® 4 Personal Microarrayers
SpotBot® Extreme Microarrayers
SpotBot® Titan Microarrayers
InnoScan® Microarray Scanners
SpotLight™ 2 Microarray Scanners
Microarray Hybridization Cassettes
High Throughput Wash Stations
Microarray High-Speed Centrifuges
Protein Printing Buffer
BlockIt™ Blocking Buffer
Microarray Air Jet
Microarray Cleanroom Wipes
Green540 and Red640 Reactive Fluorescent Dyes

