Mirror Substrates
Data Sheet
![]() Shop this product in our online store
Shop this product in our online store
Products - Microarray Substrates & Slides - Mirror Substrates for Microarray Signal Enchancement

ArrayIt® Brand Mirror Microarray Substrates are pristine, optically flat glass printing surfaces with superior coupling chemistry and a highly reflective backside surface (mirror) that increases the signal-to-noise ratio (SNR) by 2-10 fold on all fluorescent detection instruments! Fluorescent signal lost with conventional glass substrates is reflected off the backside mirror, sending signals soaring! Mirror substrates have improved SNR on every microarray scanner and imager tested including PerkinElmer, Axon and others. The mirror also allows users to see printed and hybridized arrays, which speeds array QC and increases ease of use. Mirror substrates have precise physical dimensions, are polished to atomic smoothness, and are manufactured in class 1 cleanrooms. This is the ultimate microarray surface for applications required the greatest detectivity, including gene expression monitoring and diagnostics involving weak signals, and rare mRNAs and proteins. At 25 x 76 mm, ArrayIt® Mirror Microarray Substrates are compatible with all major brands of microarrayers and scanners.
Table of Contents
- Introduction
- Quality Control
- Product Description
- Glass Specifications
- Technical Assistance
- Short Protocols
- Complete Protocols
- Technical Note
- Equipment Requirements
- Troubleshooting Tips
- Ordering Information
- Storage Conditions
- Warranty
Introduction
Congratulations on taking a big step towards improving the affordability, quality and speed of your genomics, biomedical, pharmaceutical, agricultural, and diagnostics research. This booklet contains all the information required to take full advantage of Arrayit's ArrayIt® Brand Mirror Microarray Substrates.
Quality Control
Arrayit uses rigorous quality control and quality assurance measures to guarantee the quality of our ArrayIt® Brand Mirror Microarray Substrates. The finest microarray and cleanroom-based research was used to develop these products. Rigorous quality testing on a substrate-by-substrate basis guarantees that these products conform to the highest industry standards.
Product Description
Arrayit's ArrayIt® Brand Mirror Microarray Substrates provide the highest quality reflective microarray printing substrates at an affordable price. All of our mirror substrates are manufactured in state-of-the art class 1 cleanrooms, using the most sophisticated surface deposition technology and surface chemistry in the world.
Users will appreciate the following:
- Highly reflective mirror backing
- 2-10X increase in signal-to-noise ratio (SNR)
- Improves SNR on all scanners and imagers
- Reduces background noise on all scanners and imagers
- Ideal for detecting rare mRNAs and low abundance proteins
- Manufactured in state-of-the-art class 1 cleanrooms
- Mirror backing stable to boiling and chemical treatments
- Four surfaces: plain glass, amine, aldehyde and epoxy
- Ultra-low background fluorescence
- Optimal density of reactive groups
- 5 x 10^12 reactive groups per mm^2
- Compatible with both contact and non-contact printing
- Compatible with all commercial microarray detection systems
- Precise tolerances: 25 ± 0.2 x 76 ± 0.3 x 0.960 ± 0.025 mm
- Couple nucleic acids, proteins, small molecules, and cells
- Stable surface chemistry: MirrorClean and MirrorAldehyde (12 months), MirrorAmine (6 months), and MirrorEpoxy (3 months)
Glass Specifications
The mirrors used for these substrates have been optimized for microarray applications. These are not microscope slides or conventional mirrors! Untreated mirrors of this optical quality routinely sell for $200-500 dollars each in the field.
- Standard substrate format: 25 ± 0.2 x 76 ± 0.3 x 0.960 ± 0.025 mm
- Chamfer in upper right corner allows unambiguous mirror substrate orientation
- Polished to an optical smoothness of <50 Å
- Reactive group uniformity ± 2% across surface
- Refractive index of 1.52 (400-700 nm)
- Glass transmission of >91 % (380-700 nm)
- Thermal strain point: 490°C
- Ribonuclease, protease, and DNase: None detected
- Background fluorescence (ScanArray Express at 90% laser and 90% PMT: <1,000 MirrorClean, <3,000 MirrorAmine and MirrorEpoxy, and <20,000 MirrorAldehyde
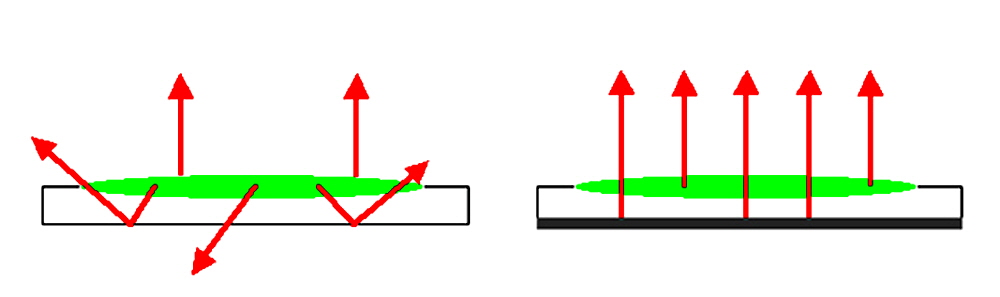
Figure 1. Comparing the optical properties of glass and mirror substrates. In the presence of excitation light, a fluorescent microarray spot (green oval) emits fluorescent signal (red arrows). A standard glass substrate (left) produces a weaker fluorescent signal and elevated noise due to the loss of excitation signal through the transparent back surface and light scattering. A mirror substrate (right) produces a stronger fluorescent signal and reduced noise due to the capture of excitation signal off the reflective backside surface and the elimination of light scattering.
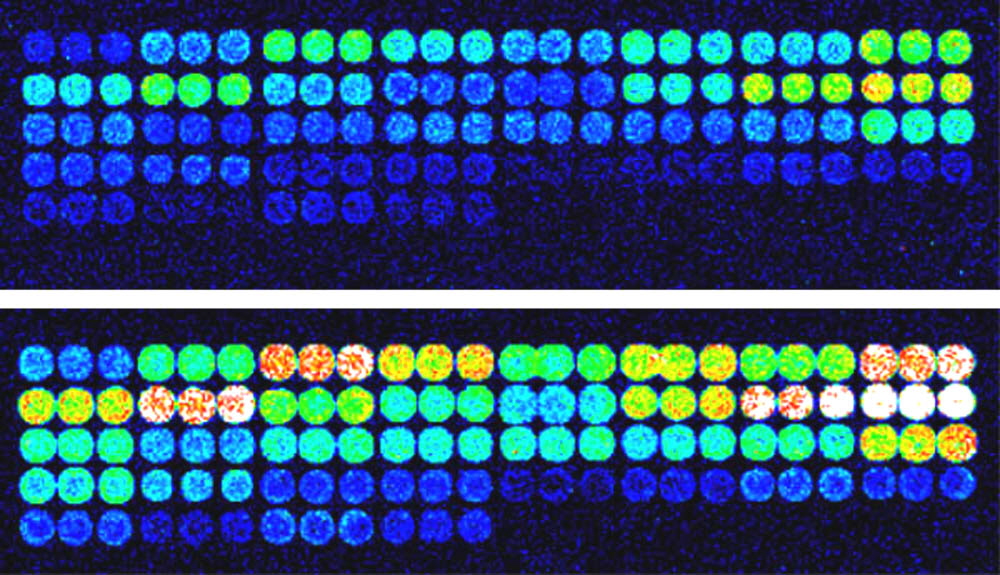
Figure 2. Mirror data. Identical oligonucleotide microarray elements were printed on SuperAldehyde (top) or MirrorAldehyde (bottom), hybridized with CheckIt Chips fluorescent probe, and scanned on a ScanArray Express at 10% laser power and 90% PMT. A 4-fold increase in signal-to-noise ratio (SNR) is obtained with the mirror surface (bottom) relative to the transparent glass substrate (top).
Technical Assistance
Please contact us if you have any comments, suggestions, or if you need technical assistance. By electronic mail: arrayit@arrayit.com (under the subject heading please type "ArrayIt® technical assistance"). By telephone: (408) 744-1331, Monday–Friday PST 8:00am - 7 pm. Please remember that we want to hear about your successes!
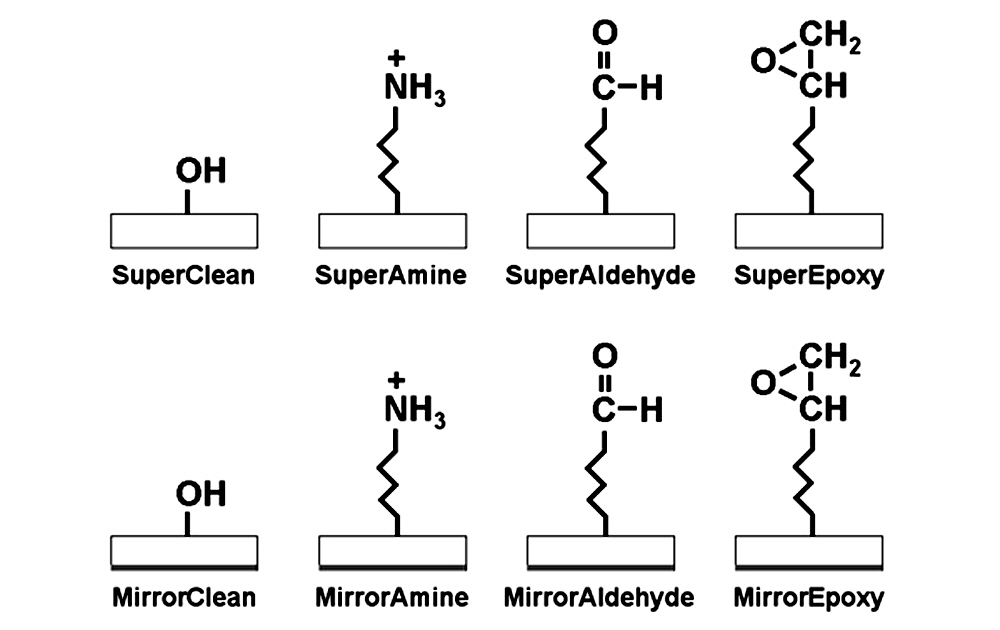
Figure 3. Coupling chemistries compared. Shown are the reactive groups present on the glass surface of SuperClean (SMC), SuperAmine (SMM), SuperAldehyde (SMA), SuperEpoxy (SME), MirrorClean (MRC), MirrorAmine (MRM), MirrorAldehyde (MRA) and MirrorEpoxy (MRE). The Super and Mirror series products are identical except that Mirror Substrates contain a highly reflective backside surface (gray bar).
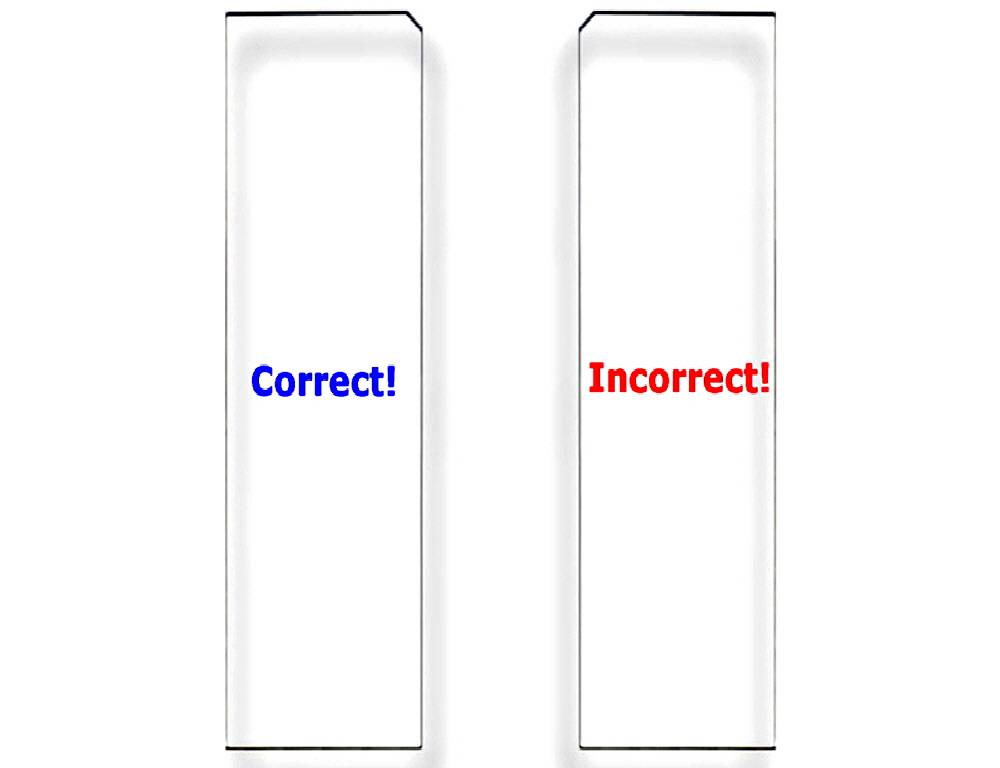
Figure 4. Correct Substrate Orientation. Shown is a graphic of two ArrayIt® Microarray Substrates, showing the correct and incorrect orientation for use. In the correct orientation (blue graphic), the chamfer will be located in the upper right corner and samples should be printed on the side facing upward, which is the same side that contains the word “Correct!”. In the incorrect orientation (red graphic), the chamfer will be located in the upper left corner, placing the backside facing upward, which is the side that contains the word “Incorrect!”. Only one side of ArrayIt® Microarray Substrates is suitable for printing. Please print on the correct side only.
Short Protocol (MirrorClean)
1. (Optional) Decontaminate the silver antistatic bag.
2. (Optional) Transport the decontaminated product into the cleanroom.
3. Remove and discard silver antistatic bag.
4. Open box containing 25 MirrorClean Microarray Substrates (MRC).
5. Modify MirrorClean Substrates with a surface chemistry or surface coating.
6. Print microarrays using Stealth contact printing technology.
Short Protocol (MirrorAmine)
1. (Optional) Decontaminate the silver antistatic bag.
2. (Optional) Transport the decontaminated product into the cleanroom.
3. Remove and discard silver antistatic bag.
4. Open box containing 25 MirrorAmine Microarray Substrates (MRM).
5. Print microarrays using Stealth contact printing technology.
6. Process the printed microarrays.
7. React microarrays with labeled probes.
8. Detect microarray signals using fluorescence detection device.
9. Analyze and model data.
Short Protocol (MirrorAldehyde)
1. (Optional) Decontaminate the silver antistatic bag.
2. (Optional) Transport the decontaminated product into the cleanroom.
3. Remove and discard silver antistatic bag.
4. Open box containing 25 MirrorAldehyde Microarray Substrates (MRA).
5. Print microarrays using Stealth contact printing technology.
6. Process the printed microarrays.
7. React microarrays with labeled samples.
8. Detect microarray signals with using fluorescence detection device.
9. Analyze and model data.
Short Protocol (MirrorEpoxy)
1. (Optional) Decontaminate the silver antistatic bag.
2. (Optional) Transport the decontaminated product into the cleanroom.
3. Remove and discard silver antistatic bag.
4. Open box containing 25 MirrorEpoxy Microarray Substrates (MRE).
5. Print microarrays using Stealth contact printing technology.
6. Process the printed microarrays.
7. React microarrays with labeled samples.
8. Detect microarray signals with using fluorescence detection device.
9. Analyze and model data.
Complete Protocol (MirrorClean)
1. (Optional) Decontaminate the silver antistatic bag. Use a Microarray Air Jet or an equivalent oil-free air stream to remove particles from the plastic packaging exterior. Wipe the outside with 100% ethanol and Microarray Cleanroom Wipes. These steps are optional and only required for users who fabricate microarrays in a cleanroom environment. Shipping material can contaminate the exterior packaging material with particulates that can be transferred into the cleanroom.
2. (Optional) Transport the decontaminated product into the cleanroom. This step is optional and only required for users who fabricate microarrays in a cleanroom environment. Carry the de-contaminated MirrorClean Microarray Substrates in the sealed plastic shipping envelope into the cleanroom changing area. Change into cleanroom attire and enter the cleanroom facility.
3. Remove and discard silver antistatic bag. Remove the antistatic packaging envelope and discard it into an appropriate waste receptacle.
4. Open box containing 25 MirrorClean Microarray Substrates (MRC or MRCBC). Use the white cleanroom wipe inside the substrate box to gently lift the cover upward. Do not attempt to open the substrate box upside down, as this will cause the Substrates to empty into the lid of the box and may damage or break the glass. Avoid dragging the cleanroom wipe across the substrates as this may cause fraying of the wipe and particle generation.
5. Modify MirrorClean Substrates with a surface chemistry or surface coating. Surface chemistries may include the use of organosilanes or coatings such as poly-lysine, gels, gel pads and other materials. If only one of the two sides of the Substrate is modified, the recommended convention places the corner chamfer (i.e. cropped corner) at the upper right if the Substrate is held with the long dimension facing up and down (see Figure 7). After the surface chemistry or coating is applied, remove access or unbound material from the Substrate. After surface modification, the Substrates can be barcoded for identification purposes (see Figure 7).
6. Print microarrays using Stealth contact printing technology or another printing technology onto the modified MirrorClean Microarray Substrates. The SpotBot® 2 Personal Microarrayer works well for many applications. Many different pin tips and uptake channels are available, allowing the user to select different spot diameters and loading volumes. For best results use Micro Spotting Solution Plus printing buffer. The printing convention places the corner chamfer at the upper right side if the Substrate is held with the long dimension facing up and down (see Figure 7). Spot number one would therefore be located at the upper left corner on the side opposite the corner chamfer (Fig. 7). A barcode could then be placed on the lower end of the Substrate, preferably facing upward (Fig. 7). Process the Substrates to remove unbound material and react with a labeled sample.
Complete Protocol (MirrorAmine)
1. (Optional) Decontaminate the silver antistatic bag. Use a Microarray Air Jet or an equivalent oil-free air stream to remove particles from the plastic packaging exterior. Wipe the outside with 100% ethanol and Microarray Cleanroom Wipes. These steps are optional and only required for users who fabricate microarrays in a cleanroom environment. Shipping material can contaminate the exterior packaging material with particulates that can be transferred into the cleanroom.
2. (Optional) Transport the decontaminated product into the cleanroom. This step is optional and only required for users who fabricate microarrays in a cleanroom environment. Carry the de-contaminated MirrorAmine Microarray Substrates in the sealed plastic shipping envelope into the cleanroom changing area. Change into cleanroom attire and enter the cleanroom facility.
3. Remove and discard silver antistatic bag. Remove the antistatic packaging envelope and discard it into an appropriate waste receptacle.
4. Open box containing 25 MirrorAmine Microarray Substrates (MRM or MRMBC). Use the white cleanroom wipe inside the substrate box to gently lift the cover upward. Do not attempt to open the substrate box upside down, as this will cause the Substrates to empty into the lid of the box and may damage or break the glass. Avoid dragging the cleanroom wipe across the substrates as this may cause fraying of the wipe and particle generation.
5. Print microarrays using Stealth contact printing technology. Print the microarrays using either Micro Spotting Pins or some other contact or non-contact printing technology. The SpotBot® 2 Personal Microarrayer works well for many applications. For best results use Micro Spotting Solution Plus printing buffer. Load the MirrorAmine Microarray Substrates onto the printing surface of a microarraying device, with the corner chamfer located at the upper right corner as shown in Figure 7. Because the highly reflective mirror coating is attached to the backside of the substrate, DO NOT attempt to print on the backside of the Substrate. The printing convention places spot number one in the upper left corner opposite the corner chamfer (see Figure 7). Print the microarrays until all of the samples have been deposited. The maximum recommended printing area is 20 mm x 72 mm, unless barcodes are used and then the printing area will be smaller.
6. Process the printed microarrays. Chemically couple the biomolecules to the MirrorAmine surface and process the printed microarrays to remove unbound material. Optimal protocols for DNA, proteins, small molecules, extracts, cells and other molecules have been developed. One protocol that works well for double-stranded nucleic acids is as follows: (1) re-suspend the double-stranded DNAs in Micro Spotting Solution Plus, (2) print the DNAs onto MirrorAmine Substrates with Stealth Micro Spotting Technology, (3) bake the printed substrates for 80 minutes at 80°C in a drying oven without vacuum, (4) rinse the printed substrates twice in 0.1% SDS and once in dH2O for 2 min each wash at room temperature (22-25°C) to remove unbound DNA, (5) denature the double-stranded DNA on the surface by immersing the substrates in boiling dH20 (100°C) for 3 min, (7) plunge the substrates into ice cold 100% ethanol for 30 sec to fix the denatured DNA, and (8) dry the processed Substrates by centrifugation for 10 sec in a Microarray High Speed Centrifuge or for 1 min at 500 x g in a tabletop centrifuge (e.g Savant).
7. React microarrays with labeled probes. React the processed microarrays with labeled probe mixtures. Probes can be complex mixtures of labeled or fluorescent nucleic acids, proteins, antibodies, extracts and other substances. Microarray reactions can be performed under glass cover slips at a volume of 2.0 µl per cm2 of cover slip. Pre-heating the probe to 42-65°C just prior to hybridization can greatly reduce background!! UniHyb™ Hybridization Solution, HybIt™ Hybridization Solution, and HybIt™ Hybridization Solution work well at a wide range of temperatures and reduce background hybridization is many cases. These buffers can also be used for single nucleotide polymorphism (SNP) analysis. Buffers consisting of 5X SSC or 6X SSPE and 0.1% SDS can also be used. The addition of 0.2 mg/ml BSA (Worthington) to the hybridization reaction can reduce background. Hybridization Cassettes provide a convenient reaction environment for all types of reactions. Following the microarray reaction, unbound probe material is removed by several successive washes in dilute buffers at room temperature (~25°C). Wash buffers should be adjusted when trying to detect short duplexes or SNPs.
8. Detect microarray signals using fluorescence detection device. Suitable detection systems accommodate the 25 mm x 76 mm (1" x 3") substrate and have pixel resolutions from 3-50 µm. Recommended scanning and imaging resolution is ~1/10 spot diameter. High quality scanners and images are available from PerkinElmer, Bio-Rad, Axon, Amersham, Applied Precision, Agilent, and others. Signal-to-noise (SNR) ratios should be 2-10 fold greater than with traditional, non-reflective glass substrates.
9. Analyze and model data. Microarray images should be saved as 16-bit Tagged Image File Format (TIFF) files for ease of quantitation and modeling. Quantitation templates can be superimposed over TIFF images for automated quantitation. Data can be normalized and transformed for greater statistical soundness when comparing multiple channels or microarrays. Scatter plots, principle component analysis (PCA), cluster analysis, self organizing maps (SOMs), expression maps and various supervised methods are among the approaches that can be used for analysis and modeling. High quality tools are available from Applied Maths, Applied Precision, Array Genetics, BioDiscovery, Compugen, Imaging Research Inc., Iobion Informatics, Lion bioscience AG, MediaCybernetics, MiraiBio Inc., PerkinElmer, PREMIER Biosoft, Rosetta Inpharmatics, Silicon Genetics, VisX Labs, and others.
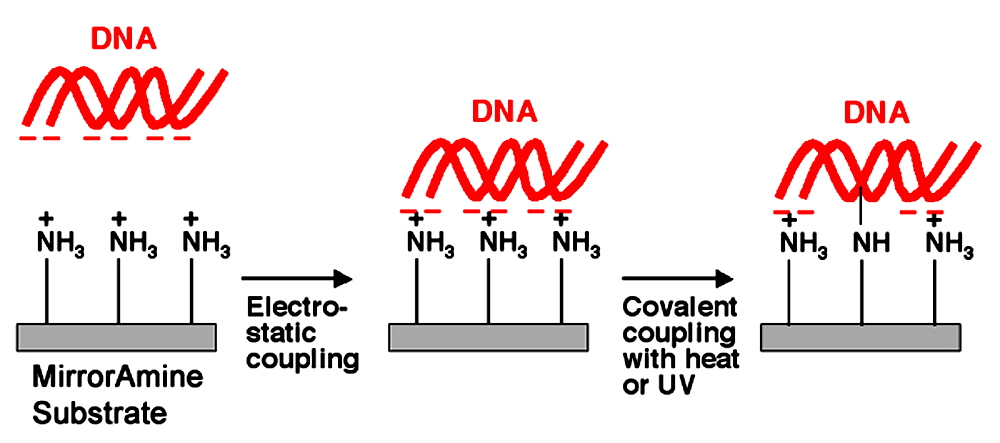
Figure 4. MirrorAmine Attachment Chemistry. MirrorAmine Substrates (rectangles) contain primary amine groups (NH3+) attached covalently to the glass surface that contains a highly reflective backside coating. The amines carry a positive charge at neutral pH, allowing attachment of native DNA (red ribbons) through the formation of ionic bonds with the negatively charged phosphate backbone (middle panel). Electrostatic attachment may be supplemented by treatment with heat or ultraviolet light, which induces covalent attachment of the DNA to the surface (right panel). The combination of electrostatic binding and covalent attachment couples the DNA to the substrate in a highly stable manner. Proteins can also be attached to MirrorAmine, though the MirrorEpoxy surface is generally recommended for protein microarrays.
Complete Protocol (MirrorAldehyde)
1. (Optional) Decontaminate the silver antistatic bag. Use a Microarray Air Jet or an equivalent oil-free air stream to remove particles from the plastic packaging exterior. Wipe the outside with 100% ethanol and Microarray Cleanroom Wipes. These steps are optional and only required for users who fabricate microarrays in a cleanroom environment. Shipping material can contaminate the exterior packaging material with particulates that can be transferred into the cleanroom.
2. (Optional) Transport the decontaminated product into the cleanroom. This step is optional and only required for users who fabricate microarrays in a cleanroom environment. Carry the de-contaminated MirrorAldehyde Microarray Substrates in the sealed plastic shipping envelope into the cleanroom changing area. Change into cleanroom attire and enter the cleanroom facility.
3. Remove and discard silver antistatic bag. Remove the antistatic packaging envelope and discard it into an appropriate waste receptacle.
4. Open box containing 25 MirrorAldehyde Microarray Substrates (MRA or MRABC). Use the white cleanroom wipe inside the substrate box to gently lift the cover upward. Do not attempt to open the substrate box upside down, as this will cause the Substrates to empty into the lid of the box and may damage or break the glass. Avoid dragging the cleanroom wipe across the substrates as this may cause fraying of the wipe and particle generation.
5. Print microarrays using Stealth contact printing technology. Print the microarrays using either Micro Spotting Pins or some other contact or non-contact printing technology. The SpotBot® 2 Personal Microarrayer works well for many applications. For best results use Micro Spotting Solution Plus printing buffer. Load the MirrorAldehyde Microarray Substrates onto the printing surface of a microarraying device, with the corner chamfer located at the upper right corner as shown in Figure 7. Because the highly reflective mirror coating is attached to the backside of the substrate, DO NOT attempt to print on the backside of the Substrate. The printing convention places spot number one in the upper left corner opposite the corner chamfer (see Figure 7). Print the microarrays until all of the samples have been deposited. The maximum recommended printing area is 20 mm x 72 mm, unless barcodes are used and then the printing area will be smaller.
6. Process the printed microarrays. Chemically couple the biomolecules to the MirrorAldehyde surface and process the printed microarrays to remove unbound material. Optimal protocols for DNA, proteins, small molecules, extracts, cells and other molecules have been developed. One protocol that works well for printing and attaching single- and double-stranded nucleic acids to the MirrorAldehyde surface uses DNA modified with primary amines that form Schiff base covalent bonds with reactive aldehydes on the Substrate surface: (1) attach a C6 or C12 amino modification (Glen Research) to the 5' end of each oligonucleotide; amino modifications can be added to double stranded DNAs by PCR with primers that contain amino modifications, (2) re-suspend the amino-linked DNAs in 1X Micro Spotting Solution Plus, (3) print the amino-linked DNAs onto MirrorAldehyde Substrates with Stealth Micro Spotting Technology, (4) allow the Substrates to dry for 12 hrs at room temperature (~25°C) at <30% relative humidity, (5) rinse the printed substrates twice in 0.1% SDS and once in dH2O for 2 min each wash at room temperature (22-25°C) to remove unbound DNA, (6) rinse the Substrates once in dH2O for 2 min at room temperature with vigorous agitation, (7) transfer the Substrates into boiling dH2O at 100°C for 3 min to denature the DNA (8) plunge the substrates into ice cold 100% ethanol for 30 sec to fix the denatured DNA, and (9) dry the processed Substrates by centrifugation for 10 sec in a Microarray High Speed Centrifuge or for 1 min at 500 x g in a tabletop centrifuge (e.g Savant).
7. React microarrays with labeled probes. React the processed microarrays with labeled probe mixtures. Probes can be complex mixtures of labeled or fluorescent nucleic acids, proteins, antibodies, extracts and other substances. Microarray reactions can be performed under glass cover slips at a volume of 2.0 µl per cm2 of cover slip. Pre-heating the probe to 42-65°C just prior to hybridization can greatly reduce background!! UniHyb™ Hybridization Solution, and HybIt™ Hybridization Solution work well at a wide range of temperatures and reduce background hybridization is many cases. These buffers can also be used for single nucleotide polymorphism (SNP) analysis. Buffers consisting of 5X SSC or 6X SSPE and 0.1% SDS can also be used. The addition of 0.2 mg/ml BSA (Worthington) to the hybridization reaction can reduce background. Hybridization Cassettes provide a convenient reaction environment for all types of reactions. Following the microarray reaction, unbound probe material is removed by several successive washes in dilute buffers at room temperature (~25°C). Wash buffers should be adjusted when trying to detect short duplexes or SNPs.
8. Detect microarray signals using fluorescence detection device. Suitable detection systems accommodate the 25 mm x 76 mm (1" x 3") substrate and have pixel resolutions from 3-50 µm. Recommended scanning and imaging resolution is ~1/10 spot diameter. High quality scanners and images are available from PerkinElmer, Bio-Rad, Axon, Amersham, Applied Precision, Agilent, and others. Signal-to-noise (SNR) ratios should be 2-10 fold greater than with traditional, non-reflective glass substrates.
9. Analyze and model data. Microarray images should be saved as 16-bit Tagged Image File Format (TIFF) files for ease of quantitation and modeling. Quantitation templates can be superimposed over TIFF images for automated quantitation. Data can be normalized and transformed for greater statistical soundness when comparing multiple channels or microarrays. Scatter plots, principle component analysis (PCA), cluster analysis, self organizing maps (SOMs), expression maps and various supervised methods are among the approaches that can be used for analysis and modeling. High quality tools are available from Applied Maths, Applied Precision, Array Genetics, BioDiscovery, Compugen, Imaging Research Inc., Iobion Informatics, Lion bioscience AG, MediaCybernetics, MiraiBio Inc., PerkinElmer, PREMIER Biosoft, Rosetta Inpharmatics, Silicon Genetics, VisX Labs, and others.
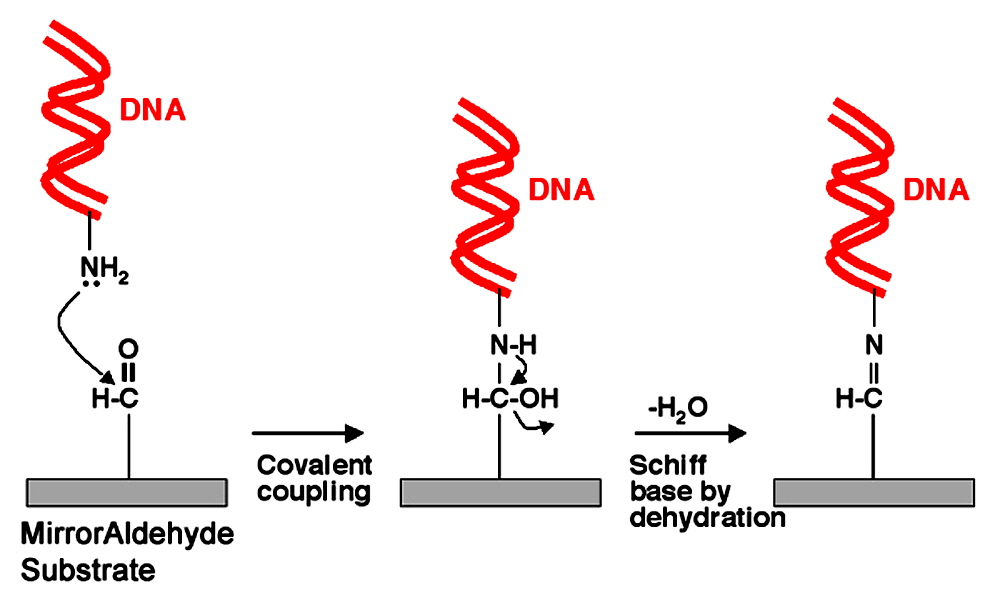
Figure 5. MirrorAldehyde Attachment Chemistry. MirrorAldehyde Substrates (rectangles) contain primary aldehyde groups attached covalently to the glass surface that contains a highly reflective backside coating. Primary amino linkers (NH2) on the DNA (red ribbons) attack the aldehyde groups (left panel) forming covalent bonds (center panel). Attachment is stabilized by a dehydration reaction (drying in low humidity) leading to Schiff base formation (right panel). Specific and cvalent end attachment provide highly stable and accessible target molecules for gene expression and genotyping applications including single nucleotide polymorphism (SNP) analysis. Proteins can also be attached to MirrorAldehyde, though the MirrorEpoxy surface is generally recommended for protein microarrays.
Complete Protocol (MirrorEpoxy)
1. (Optional) Decontaminate the silver antistatic bag. Use a Microarray Air Jet or an equivalent oil-free air stream to remove particles from the plastic packaging exterior. Wipe the outside with 100% ethanol and Microarray Cleanroom Wipes. These steps are optional and only required for users who fabricate microarrays in a cleanroom environment. Shipping material can contaminate the exterior packaging material with particulates that can be transferred into the cleanroom.
2. (Optional) Transport the decontaminated product into the cleanroom. This step is optional and only required for users who fabricate microarrays in a cleanroom environment. Carry the de-contaminated MirrorEpoxy Microarray Substrates in the sealed plastic shipping envelope into the cleanroom changing area. Change into cleanroom attire and enter the cleanroom facility.
3. Remove and discard silver antistatic bag. Remove the antistatic packaging envelope and discard it into an appropriate waste receptacle.
4. Open box containing 25 MirrorEpoxy Microarray Substrates (MRE or MREBC). Use the white cleanroom wipe inside the substrate box to gently lift the cover upward. Do not attempt to open the substrate box upside down, as this will cause the Substrates to empty into the lid of the box and may damage or break the glass. Avoid dragging the cleanroom wipe across the substrates as this may cause fraying of the wipe and particle generation.
5. Print microarrays using Stealth contact printing technology. Print the microarrays using either Micro Spotting Pins or some other contact or non-contact printing technology. The SpotBot 2® Personal Microarrayer works well for many applications. For best results use Micro Spotting Solution Plus printing buffer. Load the MirrorEpoxy Microarray Substrates onto the printing surface of a microarraying device, with the corner chamfer located at the upper right corner as shown in Figure 7. Because the highly reflective mirror coating is attached to the backside of the substrate, DO NOT attempt to print on the backside of the Substrate. The printing convention places spot number one in the upper left corner opposite the corner chamfer (see Figure 7). Print the microarrays until all of the samples have been deposited. The maximum recommended printing area is 20 mm x 72 mm, unless barcodes are used and then the printing area will be smaller.
6. Process the printed microarrays. Chemically couple the biomolecules to the MirrorAmine surface and process the printed microarrays to remove unbound material. Optimal protocols for DNA, proteins, small molecules, extracts, cells and other molecules have been developed. One protocol that works well for single- or double-stranded DNA is as follows: (1) Suspend DNA samples in Micro Spotting Solution Plus by mixing 1 volume of 0.2-1.0 µg/µl cDNA products or 60-100 µM oligonucleotides and 1 volume of 2X Micro Spotting Solution Plus, (2) mix the samples by pipetting up and down 10 times, (3) print DNA samples onto MirrorEpoxy Substrates, (4) couple DNA molecules to the surface by incubating for 10 minutes at room temperature (25°C), (5) just prior to use, wash the printed microarrays to remove unbound DNA molecules and printing buffer components from the substrate as follows: wash 2 min at room temperature in 2X SSC + 0.1% sarcosyl, wash 2 min at room temperature in 2X SSC, denature DNA for 2 min at 100°C in dH2O, cool 10 sec at room temperature, and fix by plunging for 2 min in ice cold 100% ethanol.
The processed microarrays can be washed using a High Throughput Wash Station and dried using a Microarray High-Speed Centrifuge. Protein microarray applications require different protocols than DNA microarrays. One protocol that works well for protein microarrays is as follows: (1) suspend protein samples in Protein Printing Buffer by mixing 1 volume of 0.2-1.0 µg/µl protein sample in 1X phosphate buffered saline (PBS) and 1 volume of 2X Protein Printing Buffer, (2) mix the samples by pipetting up and down 10 times, (3) print protein samples onto MirrorEpoxy Substrates, (4) couple protein molecules to the surface by incubating for 10 minutes at room temperature (25°C), (5) just prior to use, remove unbound protein molecules and printing buffer components by washing the substrates 3 times for 5 min each at room temperature in 1X PBS, (6) block the surface by incubating the substrates for 30 min at room temperature in 1X PBS + 1% bovine serum albumin (BSA), (7) remove excess BSA by washing three times for 2 min each at room temperature in 1X PBS. Washes should be performed using gentle agitation in a High Throughput Wash Station. Drying can be performed by tapping the processed substrates on a Microarray Cleanroom Wipe or by using a Microarray High-Speed Centrifuge.
7. React microarrays with labeled probes. React the processed microarrays with labeled probe mixtures. Probes can be complex mixtures of labeled or fluorescent nucleic acids, proteins, antibodies, extracts and other substances. Microarray reactions can be performed under glass cover slips at a volume of 2.0 µl per cm2 of cover slip. Protein microarray reactions can be performed under cover slips, as a droplet on the printed microarray, or in a microfluidics chamber. For DNA and protein microarrays, 1-12 hour reactions are sufficient to obtain intense fluorescent signals. Pre-heating DNA probes to 42-65°C just prior to hybridization can greatly reduce background, though protein mixtures should not be pre-heated beyond 37°C. For nucleic acid applications, UniHyb™ Hybridization Solution, and HybIt™ Hybridization Solution work well at a wide range of temperatures and reduce background hybridization is many cases. These buffers can also be used for single nucleotide polymorphism (SNP) analysis. Buffers consisting of 5X SSC or 6X SSPE and 0.1% SDS can also be used. The addition of 0.2 mg/ml BSA (Worthington) to the hybridization reaction can reduce background. For protein and antibody reactions, binding reactions can be performed in 1X PBS + 0.5% BSA + fluorescent cellular extract or antibodies diluted 1:1,000. Protein fluorescent labeling reagents are available from a large number of vendors. Hybridization Cassettes provide a convenient reaction environment for all types of reactions. Following microarray hybridization and protein binding reactions, unbound fluorescent probe material must be washed away. For cDNA microarrays, the following wash protocol works well: 5 min at room temperature with 2X SSC + 0.1% sarcosyl, 5 min at room temperature with 0.2X SSC + 0.1% sarcosyl, and 1 min at room temperature in 0.2X SSC. For oligonucleotide microarrays, the following wash procedure works well: 5 min at room temperature with 2X SSC + 0.1% sarcosyl, 5 min at room temperature with 2X SSC, and 10 sec at room temperature in 0.2X SSC. For protein microarrays, the following wash procedure works well: three times for 5 min each at room temperature in 1X PBS. DNA and protein washes can be performed using a High Throughput Wash Station, and drying by spinning in a Microarray High-Speed Centrifuge. Excess wash buffer can also be removed from the surface by tapping the substrates on a Microarray Cleanroom Wipe.
8. Detect microarray signals using fluorescence detection device. Suitable detection systems accommodate the 25 mm x 76 mm (1" x 3") substrate and have pixel resolutions from 3-50 µm. Recommended scanning and imaging resolution is ~1/10 spot diameter. High quality scanners and images are available from PerkinElmer, Bio-Rad, Axon, Amersham, Applied Precision, Agilent, and others. Signal-to-noise (SNR) ratios should be 2-10 fold greater than with traditional, non-reflective glass substrates.
9. Analyze and model data. Microarray images should be saved as 16-bit Tagged Image File Format (TIFF) files for ease of quantitation and modeling. Quantitation templates can be superimposed over TIFF images for automated quantitation. Data can be normalized and transformed for greater statistical soundness when comparing multiple channels or microarrays. Scatter plots, principle component analysis (PCA), cluster analysis, self organizing maps (SOMs), expression maps and various supervised methods are among the approaches that can be used for analysis and modeling. High quality tools are available from Applied Maths, Applied Precision, Array Genetics, BioDiscovery, Compugen, Imaging Research Inc., Iobion Informatics, Lion bioscience AG, MediaCybernetics, MiraiBio Inc., PerkinElmer, PREMIER Biosoft, Rosetta Inpharmatics, Silicon Genetics, VisX Labs, and others.
Technical Note
DNA and protein samples should be free of aggregates and particulates that can clog printing devices and impair attachment to the Mirror Microarray Substrates. Aggregates and particulates can be removed by purification, centrifugation or filtration. At 30% coupling efficiency, a 30 µM oligonucleotide will produce a target density of approximately 2 x 1011 oligonucleotides per mm2 of substrate. A 90 µm microarray spot contains approximately 1.6 x 109 oligonucleotide molecules. A 50kD protein at 1 µg/µl concentration has a concentration of 20 µM. At 30% coupling efficiency, a 20 µM protein will produce a target density of 1011 proteins per mm2 of substrate. Certain proteins or protein extracts are more stable at 4°C. Keeping the protein samples cool may improve protein stability. Stability can also be improved in some cases by the addition of protease and phosphatase inhibitors and by the use of a SpotBot® 2 Personal Microarrayer equipped with a cooled platen. Make sure protein samples are mixed thoroughly before printing. On MirrorEpoxy Substrates, DNA and protein coupling is complete 10 minutes after printing, and target molecules retain their capacity to bind to fluorescent probe molecules. Drying, dehydration, blocking and cross-linking is not required with MirrorEpoxy Substrates, and may actually reduce signal intensity. Highly reactive epoxide groups on MirrorEpoxy enable extremely efficient coupling, and this surface may not be suitable for short oligonucleotides. MirrorAmine and MirrorAldehyde may work better than MirrorEpoxy for oligonucleotide applications. Printed microarrays should be stored unprocessed to protect coupled molecules from drying and oxidative damage. Micro Spotting Solution Plus and Protein Printing Buffer contain components that stabilize printed DNA and protein. Processing or printed microarrays should be performed just prior to use for best performance.
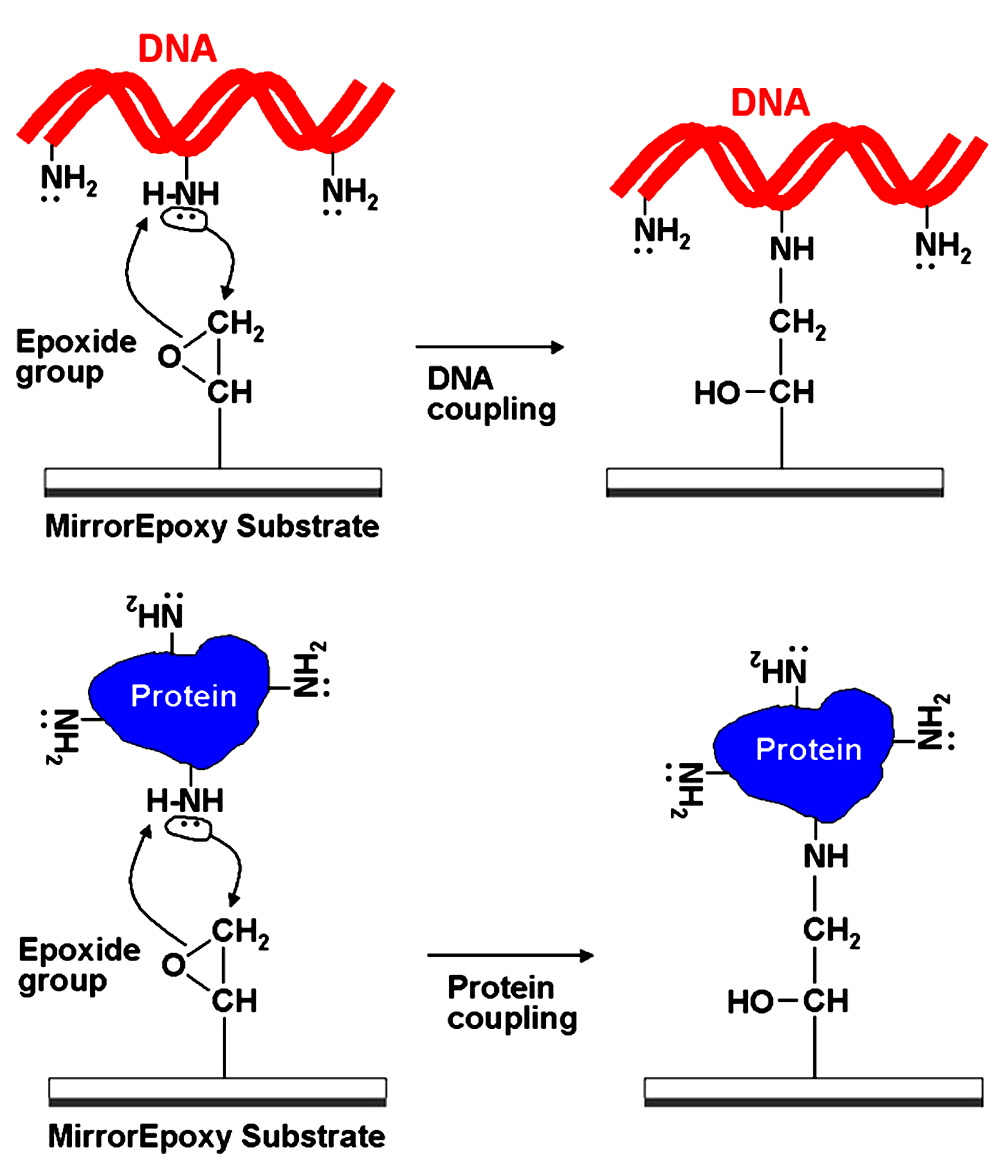
Figure 6. MirrorEpoxy Coupling Chemistry. (Top) Shown is a schematic representation of a DNA molecule (red ribbon) coupling to a MirrorEpoxy Substrate (rectangle). Oligonucleotides, cDNAs and RNAs contain primary amine groups on the A, G, and C residues. The lone electron pair (double dots) attack the electrophilic carbon on the epoxide group (arrow), forming a covalent bond (right panel) between the DNA and the substrate. (Bottom) Shown is a schematic representation of a protein molecule (blue shape) coupling to a MirrorEpoxy Substrate (rectangle). Proteins, antibodies, polypeptides, and peptides contain primary amine groups on lysine and arginine residues. The lone electron pair (double dots) attack the electrophilic carbon on the epoxide group (arrow), forming a covalent bond (right panel) between the protein and the substrate.
The “substrate noise” is the sum of all non-sample and non-instrument contributions to the background reading including intrinsic fluorescence of the substrate and reflection off the substrate surface. Because substrate noise is measured using a scanning or imaging device, as a practical matter substrate noise typically includes intrinsic fluorescence and reflection, as well as all of the sources of instrument noise (e.g. dark current, shot noise, electronic noise and optical noise). Most modern scanners and imagers have very low instrument noise, which means that intrinsic fluorescence and reflection dominate the substrate noise reading. Substrate noise is obtained by measuring the fluorescent reading of the substrate “right out of the box” and prior to reacting the substrate with a fluorescent sample. Please do not confuse substrate noise (i.e. background before the substrate is reacted with a sample) and microarray noise (i.e. background after the substrate has been reacted with a sample), as these two readings are very different. For nearly all applications and assays, microarray noise greatly exceeds substrate noise and therefore concerns about substrate noise are entirely academic because the latter does not contribute to the total background reading observed when the product is used in real experiments (see below).
The table below summarizes the substrate noise (actually intrinsic fluorescence + reflection + instrument noise) observed with our substrates. Substrate noise readings were taken at very high sensitivity (90% laser and 90% photomultiplier tube or PMT), settings that are well beyond those used for biological experiments. Typical instrument settings for biological experiments with the ScanArray Express are 20-40% laser power and 80% PMT. The ScanArray Express has 20- to 30-fold greater sensitivity than the ScanArray 3000, and correspondingly the substrate noise readings are up to 30-fold higher on the Express compared to the 3000. Unmodified glass substrates (i.e. SuperClean) produce the lowest substrate noise readings, followed SuperEpoxy, SuperAmine, and SuperAldehyde. Organic treatments (e.g. epoxy, amine and aldehyde) increase substrate noise compared to naked glass because organic molecules formed during derivation fluoresce at an extremely low but detectable level. For nearly all applications and assays, the non-sample contributions to background noise (intrinsic fluorescence, reflection, and instrument noise) are much less than the background noise contributed when the substrate or microarray is reacted with a fluorescent sample. In these cases, substrate noise (though it exists) is irrelevant to the use of our products, because it does not contribute in any measurable way to the background reading of the reacted chip. In rare cases involving extremely low background samples or gene expression measurements of rare transcripts, substrate noise may approach sample noise in magnitude. In these cases, it may be desirable to use a substrate that has a lower intrinsic fluorescence and reflection, such as SuperAmine instead of SuperAldehyde.
For best results, please test our products in the context of REAL EXPERIMENTS rather than simply taking note of the fact that our Substrates manifest substrate noise that is greater than plain glass. ALL SUBSTRATES that contain an organic treatment or coating will produce some intrinsic fluorescence and reflection. Please also test our product “right out of the box” rather than waiting hours or days to measure the substrate noise. Fluorescent contaminants present in non-cleanroom air including cleaning agents, solvents used in marking pens, and hydrocarbon emissions from vacuum pumps, arrayers, centrifuges and other instruments can elevate the substrate noise reading considerably. Please also note that airborne particles including dust and other particulates greatly elevate the background reading because these particles are highly reflective in the presence of laser and white excitation light. Understanding the technical details of our products is important and we recommend that you commit these concepts to working memory as you proceed with your experiments.
Table. Substrate noise
Product |
Average substrate noise (ScanArray 3000) |
Maximum allowable substrate noise (ScanArray 3000) |
Average substrate noise (ScanArray Express) |
Maximum allowable substrate noise (ScanArray Express) |
SuperClean |
88 |
200 |
501 |
1,250 |
MirrorClean |
63 |
150 |
377 |
950 |
SuperEpoxy |
157 |
250 |
456 |
1,500 |
MirrorEpoxy |
114 |
475 |
348 |
1,125 |
SuperAmine |
229 |
500 |
861 |
2,000 |
MirrorAmine |
168 |
375 |
636 |
1,500 |
SuperAldehyde |
278 |
500 |
729 |
2,000 |
MirrorAldehyde |
217 |
375 |
568 |
1,500 |
Troubleshooting Tips
1. Weak fluorescent signal
- Poor coupling of printed material to Mirror Substrate
- Use of hygroscopic buffers such as DMSO
- Poor labeling of probe sample
2. High background fluorescence
- Drying of labeled sample during reaction or washes
- Non-optimal reaction or wash conditions

Figure 7. Photograph of a SuperAmine Barcoded Substrate (left) and MirrorAmine Barcoded Substrate (right). Both substrates have a corner chamfer in the upper right corner for unambiguous side orientation. The chemistry and the barcode are on the front side of these substrates facing forward, whereas the non-chemistry and reflective mirror surfaces are on the backside of the Substrates shown here. Please DO NOT attempt to print on the backside of any Arrayit Substrate!
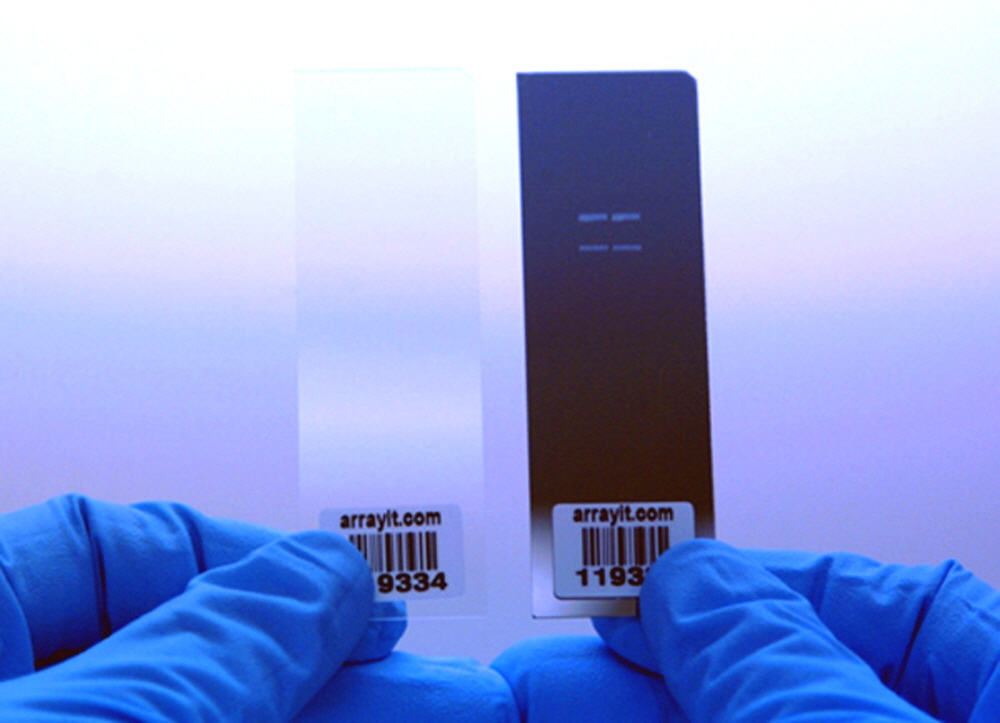
Figure 8. Photograph of identical microarrays printed on a SuperAldehyde Barcoded Substrate (left) and a MirrorAldehyde Barcoded Substrate (right). Both substrates have a corner chamfer in the upper right corner for unambiguous side orientation. Printed spots are much more visible on Mirror Substrates than on Super Substrates.
Scientific Publications
Click here and here for recent scientific publications using ArrayIt® brand microarray products from Arrayit International, Inc for mirror-based experimentation.
Recommended Equipment and Reagents
NanoPrint™ 2 Microarrayers
SpotBot® 4 Personal Microarrayers
InnoScan® Microarray Scanners
SpotLight™ 2 Microarray Scanners
Microarray Hybridization Cassettes
High Throughput Wash Station
Microarray High-Speed Centrifuge
Protein Printing Buffer
BlockIt™ Blocking Buffer
Microarray Air Jet
Microarray Cleanroom Wipes
PCR Purification Kits
SuperProtein Blocking Buffer
Micro-Total RNA Extraction Kit
MiniAmp mRNA Amplification Kit
Indirect Amino Allyl Fluorescent Labeling Kit
Universal Reference mRNA
Green540 and Red640 Reactive Fluorescent Dyes
Hybridization Buffers

