Hybridization Stations
Data Sheet
![]() Shop this product in our online store
Shop this product in our online store
Arrayit | Microarray hybridization stations TrayMix TMHS chaotic advection digital temp control mixing life sciences research
TrayMix S4 Users Manual
TrayMix S4 Installation Manual
TrayMix S4 Software Manual
Instruments - Microarray Processing - TrayMix™ S4 Automated Microarray Hybridization Stations

Arrayit TrayMix™ S4 Microarray Hybridization Stations accelerate hybridization kinetics by as much as 90% using innovative micro-mixing chaotic advection technology, and enhance automation workflow with a capacity of 4 slides per instrument and 16 slides per computer workstation. Excellent experimental results are ensured by completely homogeneous dispersion of the probe molecules across the 21 x 60 mm reaction area. TrayMix™ S4 significantly reduces hybridization time while offering reproducible and robust results from one experiment to the next using as little as 5 pmole of labeled material in the hybridization mixture. The system is easy to use and maintain, and requires no expensive gaskets or special cover slips. Greater specificity of hybridization is achieved while reducing the coefficients of variation (CV). Enhance diverse life sciences applications including gene expression, protein expression profiling, mutation detection, comparative genomic hybridization, genotyping, and FISH.
Click here to download the TrayMix™ S4 Quick Installation Guide.
Click here to download the TrayMix™ User Manual.
Review the current micro fluidic mixing techniques and see the video of the TrayMix™ Mixing loop in action at:
http://arrayit.blogspot.com/2008/10/microarray-hybridization.html
Current micro-fluidic mixing technologies that are being implemented in the microarray industry include, Turbulent Flow, Rotary Mixing with air bubbles, Laminar flow, Acoustic waves, Surface Acoustic Waves, and Chaotic Mixing. The goal of mixing a microarray binding reaction is to assure that every molecule in solution finds its binding partner immobilized on the microarray as quickly as possible. In other words, the most desirable binding reaction has fast and complete diffusion of all biomolecules in solution over all microarray spots and remains a homogeneous mixture until the reaction is complete. The chaotic mixing method of the TrayMix is superior.
Sound microarray data can be achieved using inexpensive hybridization cassettes, however, in certain cases active mixing has been shown to speed up binding reactions, improve data quality, and reduce the number of molecules required in solution for the binding or hybridization reaction. Reasons to use the TrayMix for microarray binding reactions include:
- Save time and money by performing tests faster with less test sample
- Minimize handling of microarrays, which reduces the possibility of human error.
- Get better control over the experimental variables, resulting in increased reproducibility
- Empower users to define, edit and store individual methods and protocols
- Save and link experimental or testing procedures to database
In a turbulent flow system, the hybridization cocktail is mixed by the using random contact with the physical structure of a reaction chamber. One challenge of this type of system is obtaining homogeneity of the reaction mixture.
Rotary mixing with air bubbles is performed inside a sealed reaction cassette by rotating a trapped air bubble over the microarray. Binding reactions cannot take place in air, only in solution. Therefore a challenge of this approach is minimizing air bubble-based oxidization of the fluorescent dyes commonly used in microarray reactions, which would lead to lower signals and elevated background. Another challenge is that if an air bubble were to become trapped, the reaction in the trapped area would not proceed.
Laminar Flow is generated by using a small diaphragm pumping system at each end of the microarray to move the binding reaction sample back and forth across the microarray. Recent micro-fluidic studies show that laminar flow mixing can produce layers of liquid that flow over top of each other, thus one challenge of this approach is to obtain fully homogeneous and complete mixing of sample during the laminar flow process.
Surface Acoustic Waves generated by piezoelectric transducers are used to cause streaming of the hybridization reactions under cover slips or lifter slips. Some of the same challenges that apply to laminar flow also apply to systems that use surface acoustic waves.
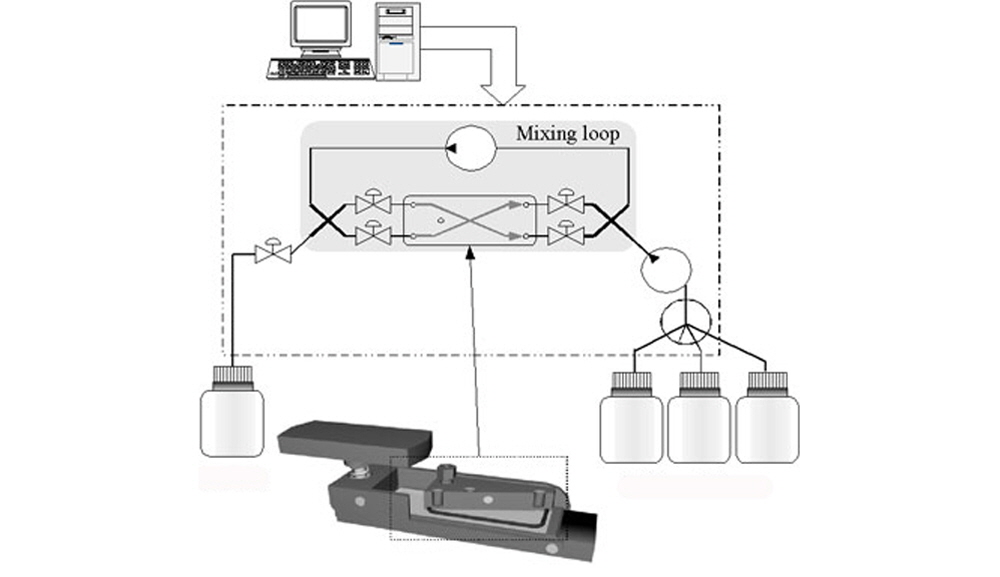
Chaotic Advection Mixing used by the TrayMix is accomplished using micro fluidic pumps and a mixing loop. The overall movement of liquid in properly configured systems is chaotic due to the extremely complex direction and speed of fluids provided by the mixing loop. This type of system provides the most complete mixing of low volumes of liquid in the shortest amount of time.
Specifications
The hybridization temperature is controllable from 20-100°C +/-0.1°C. Upstream of the reaction area, which includes the mixing loop and hybridization chamber, a manifold introduces a programmable 5-way reagent selection system. Pre-hybridization, hybridization buffer, washing solutions and decontamination solution are all computer controlled and programmable. Substrate slide drying is achieved after hybridization and washing using the ArrayIt® High-Speed Centrifuge, which dries the substrate slide completely in less than 10 seconds in preparation for microarray scanning.
Dimensions (LxWxH) |
13.7 x 18.3 x 8.3 in (35 x 26.5 x 14 cm) |
Weight |
19 kg |
Capacity |
4 standard glass substrate slides running individual or simultaneous mode per module, and 16 slides running individual or simultaneous mode per module per computer using a 4-module (4 x 4) system |
Microarray substrate |
1 x 25 x 76 mm |
Hybridization area |
21 x 60 mm |
Hybridization volume |
Hybridization chamber: 60 µl |
Hybridization reaction chamber |
Specifically designed to maintain consistent reaction volumes from hybridization to hybridization using a shim system. Rubber gaskets are used to seal the chamber, but the gaskets do not define the volume of the hybridization reaction. |
Biological samples |
Optimal quantity: 5 to 50 pmol |
Temperature range |
20-100°C +/-0.1°C |
Buffers and solutions |
Chemically-resistant to all standard biochemical reagents |
Programming |
PC + BioTray software in a windows operating system. Four stations can be run using a single computer. |
Flexibility |
Automation through computer control and easy to use software. The injection port is directly connected to the chamber allowing specific procedures to be implemented including multiple hybridizations, sandwich assays, and enzymatic reactions. |
Repeatability |
The computer controlled system performs repeatable tasks much more precisely than manual operations. |
Complete automation |
Homogeneous mixing in a controlled reaction. Programmed cycles perform pre-hybridization, hybridization, up to three different wash steps, and complete cleaning of the system in preparation for the subsequent hybridization. |
Cost effective |
No expensive cover slips and very easy to maintain. Affordable supporting microarray products from ArrayIt®, the leading brand name in the industry for more than a decade. |
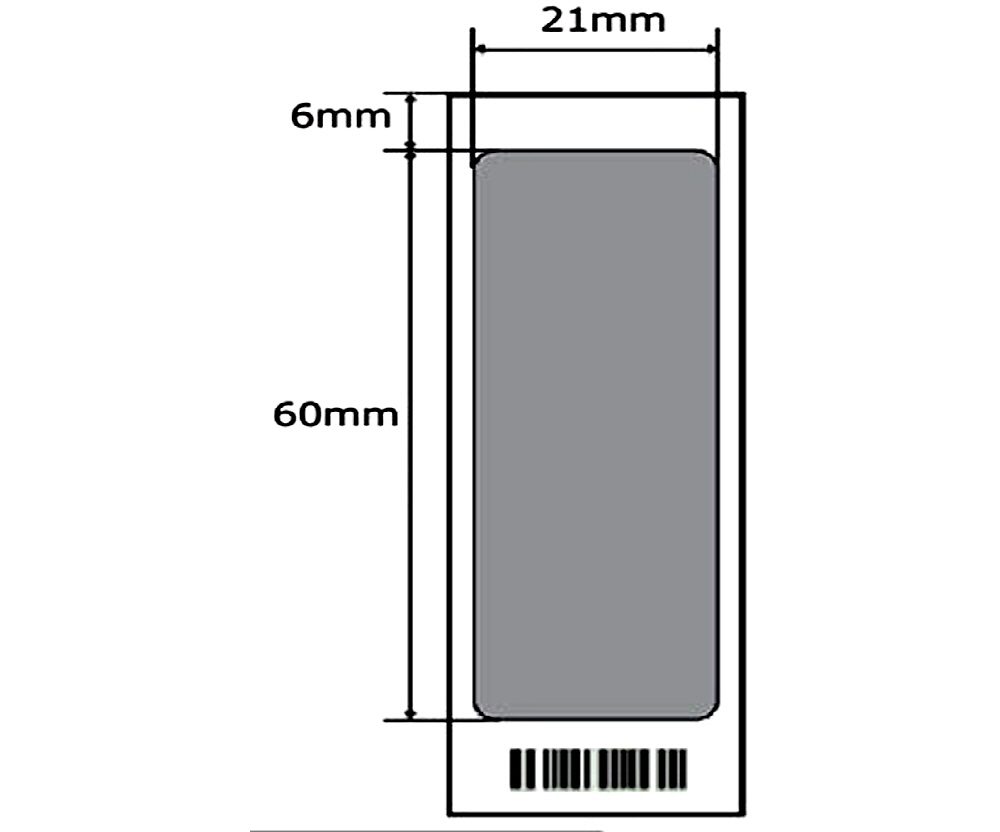
TrayMix S4 Hybridization Area.

Each hybridization chamber is totally independent. They can be launched simultaneously or programmed to run different protocols using an easy to use graphical user interface.
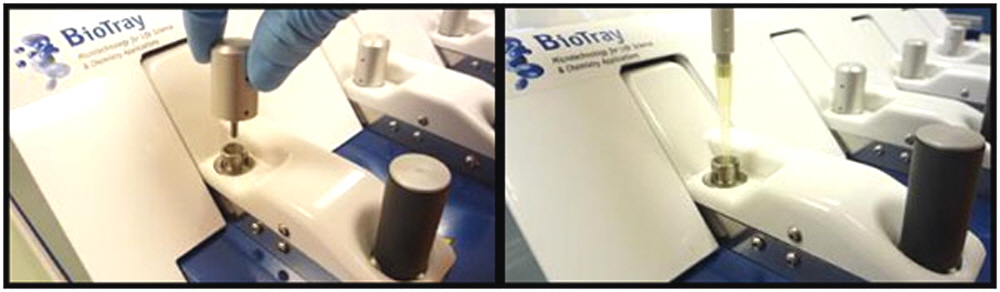
Once the system is programmed and the microarray substrate slides are in place on the platform, the system automatically delivers solutions into the hybridization chambers. The 50 µl hybridization chambers are sealed using gaskets located on the upper portion of the lids that each microarray when they are in the closed and locked position.
Up to 60 µl of sample is manually introduced into each hybridization chamber via a dedicated access port. During hybridization, the ports are closed to prevent evaporation.
Software
System programming is achieved via the easy to use graphical user interface shown below
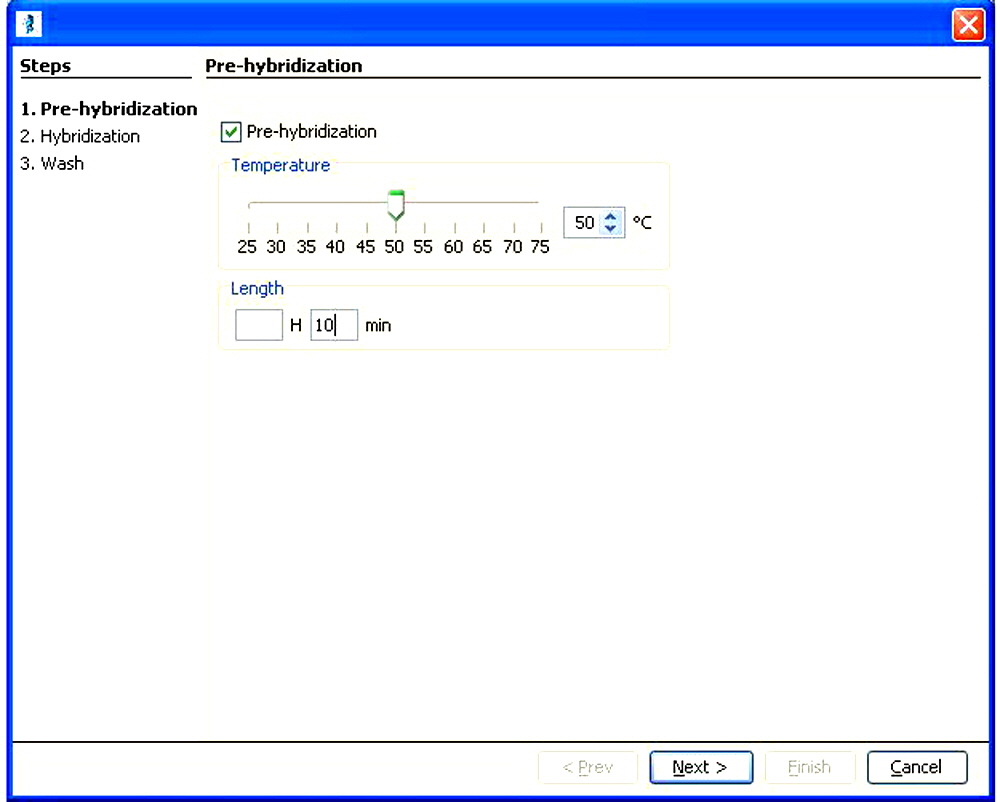
Set Pre-hybridization Time and Temperature
Additional Software Programming Steps
Set Hybridization Time and Temperature
Set Wash Parameters, program is ready to run!
Program Execution
Insert microarray slide, click proceed.
Check the system is locked properly and port cap is properly closed.
Programmed Pre-hybridization Program Starts, is useful to pre-hybridization to get the microarray at proper temperature and to wet the microarray prior to adding the labeled sample.
After Pre-hybridization is completed, temperature is stabilized, the next step is to remove air bubbles for the chaotic advection mixing loop.
Air bubbles are automatically removed.
Injecting the sample is done with a volume of 30ul or 60 ul, set volume, inject sample and click proceed.
Depending on the volume if injection, the mixing loop automatically compensates for the volume injected for hybridization.
Hybridization proceeds at set time and temperature, with constant mixing.
Wash steps proceed automatically based on set program parameters.
When chamber is empty, microarray is removed form the system and dried in a microarray centrifuge.
Software Users Manual
Click here to download the TrayMix™ S4 Software Manual.
The software interface provides several functions:
- Traceability of operations and generation of reports in HTML format
- Saving and loading of protocols
- Flexibility to modify the operation of the system in all of the experimental steps including pre-hybridization, hybridization and washing.
Comparison of chaotic advection versus traditional static hybridization:
Tables 1 and 2 below compare the performance of the TrayMix™ S2 system with static cover slip hybridization. Better signal intensity is achieved in 2 hours using the TrayMix™ S2 than in 12 hours using static coverslip hybridization.
Table 1. Average fluorescence values and coefficients of variation (CVs) measured under different hybridization conditions (2 hours of hybridization).
Probe concentration (0.1 µM) |
Solution volume (µl) |
Probe quantity(pmoles) |
Method |
Hybridization solution |
Fluorescent Mean (a.u.) |
CV |
Static Hybridization |
50 |
5 |
cover slip |
homogenous |
5500 |
0.33 |
500 |
50 |
TrayMix™ S2 |
homogenous |
38500 |
0.56 |
|
Dynamic Hybridization |
500 |
50 |
TrayMix™ S2 |
homogenous |
11474 |
0.18 |
500 |
50 |
TrayMix™ S2 |
non homogeneous |
11823 |
0.17 |
Table 2. Average value for fluorescence and CV obtained with the same quantity of targets, both with and without agitation (2 hours of hybridization).
Probe quantity (5 pmole) |
Solution volume (µL) |
Concentration (µM) |
Method |
Hybridization solution |
Fluorescence mean (a.u.) |
CV |
Static hybridization |
50 |
0.1 |
cover slip |
Homogenous |
5500 |
0.33 |
Dynamic hybridization |
500 |
0.01 |
TrayMix™ S2 |
Non-homogeneous |
6452 |
0.16 |
Signal/Noise vs Specificity
Microarrays hybridized for two hours with a single-stranded CY3 labeled DNA (84 mers) complementary to allele a. Hybridizations were performed with 5 pmol of target under the coverslip method and with the TrayMix™. Allele b was used as a control for hybridization specificity. All spot features were analyzed and compared to the local background signal for each of the experiments. Results comparing the mean signal to noise ratio of the fluorescent CY3 signals to the local background for allele a (black) and allele b (grey) of three experiments ± SD (Standard Deviation).
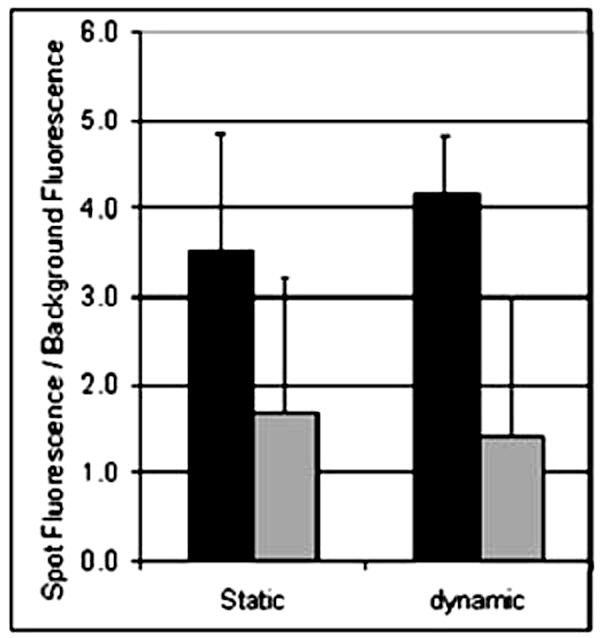
The figure shows that the specific signal/noise ratio (allele a – black bars) is enhanced from 3.5 to 4.2, while the non-specific signal/noise ratio (allele b - grey bars) is slightly reduced under the dynamic hybridization conditions of the TrayMix™. Comparisons of results after 12 hours of static hybridization are even more favorable to TrayMix™ (Data not shown). Chaotic mixing enhances the hybridization results in all important areas including time, homogeneity, and signal to noise ratio. In addition, the automation of this step is crucial for reproducibility. The system has thus been developed in view of attaining the levels of reproducibility essential for diagnostic purposes. Chaotic advection offers an even more significant advantage to passive hybridization when using high molecular weight molecules in applications such as Microarray Comparative Genomic Hybridization (aCGH).
Conclusion and perspectives
TrayMix offers a true innovation for enhancing hybridization efficacy, sensitivity, reproducibility and robustness while easy to use and flexible. The injection port is designed to allow small amounts of new reagents to be automatically and sequentially administered for pre- or post-hybridization processing
(e.g. enzymatic reactions, chemical reactions, sandwich reactions, etc…).This permits conception of more complicated processes such as oligo elongation and multi-step reactions. Furthermore, the technology allows adaptation to either multiple slides or multiple reaction areas per slide. It is to be noted that the TrayMix technology is applicable to all types of microarrays such as, CHIP on Chip, protein and peptide, CGH, and FISH. This vast spectrum of applications makes the TrayMix™ a great investment of anyone processing microarrays.
Technical Support
Please direct technical questions to arrayit@arrayit.com or email arrayit@arrayit.com.

Arrayit TrayMix™ S4 Microarray Hybridization Station including continuous power supply, BioBlue™ personal computer, 21” LED display, keyboard, mouse and mouse pad installed in a top laboratory in India.

