Genetic Disease Analysis, Biomarker Discovery, and Therapeutic Protein Validation using Advanced Microarray Technology and Bioinformatics
Data Sheet
![]() Shop this product in our online store
Shop this product in our online store
Arrayit offers full service genetic disease analysis, biomarker discovery, and therapeutic protein validation using the company’s proprietary genomic, transcriptomic, and proteomic profiling core competencies.

Arrayit offers full service genetic disease analysis, biomarker discovery, and therapeutic protein validation using the company’s proprietary genomic, transcriptomic, and proteomic profiling core competencies. Phase I, II, III, IV and V programs provide biomarker leads, biomarker scale-up studies, and FDA approvable data for 100, 500 and 1,000 samples, respectively, for any physiological pathway, drug response or disease state. Arrayit’s unique ability to quantitatively measure mRNA expression levels for all human genes, and to enhance the mRNA data with the ability to analyze variations in DNA sequences on a massive scale and interrogate protein samples for protein biomarkers of interest, offers leading edge biological information to customers not obtainable from any other source. Genome Pathway Builder™ analysis provides detailed biological pathways for disease analysis, small molecule drugs and therapeutic proteins. This service is highly recommended for complex disease states, orphan diseases and newly discovered genetic illnesses.
Contents
- Introduction
- Quality Control
- Services Description
- Program Phases I, II, III, IV and V
- Biomarker Platforms
- Technical Assistance
- Ordering Information
- Warranty
Introduction
Arrayit offers premium biomarker discovery and validation services to improve the precision, speed and affordability of genomics, transcriptomics, and proteomics information for customers in the life sciences, personal genomics, pharmaceutical, and diagnostics sectors. This handbook contains all the information required to take full advantage of Arrayit's Phase I, II, III, IV and V programs.
Quality Control
Arrayit uses the highest quality control (QC) and quality assurance (QA) procedures to ensure the quality and accuracy of the information provided in our biomarker discovery and validation services. The finest science and engineering was used to develop our transcriptomic, genomic and proteomic services products. The most advanced bioinformatics ensure that the information provided in our Phase I, II, III, IV and V programs exceeds the highest industry standards.
Services Description
Arrayit Biomarker Discovery and Validation Services offers customers the opportunity to identify biomarkers for human diseases, physiological conditions, drug pathways and other biological pathways that causes changes in gene expression. Expression profiling on samples of interest is performed using Arrayit’s sophisticated H25K Whole Human Genome Chip, the world’s only genome profiling platform designed using the entire human genome as the blueprint. Phase I, II, III, IV and V programs can be supplemented with value added genotyping and protein profiling information.
Customers will appreciate the following service features:
- Decipher the molecular basis of any human disease
- Explore any physiological pathway in human cells
- Elucidate the molecular mechanisms of small molecule drug action
- Discover and benchmark novel and existing therapeutic proteins
- Identify off-targets of drug toxicity
- Obtain validated biomarkers with a market value of >$60M
- Genome Pathway Builder™ analysis provides biological pathways for disease and drug studies
- Speed biomarker discovery
- Develop more efficacious and safer medicines
- Establish a biomarker panel for in vitro diagnostics
- Sample-to-data consulting guarantees valid results
- Comprehensive human genome content ensures complete marker set
- Proprietary two-color process affords “bullet-proof” expression markers
- Enhance marker panel with genotyping and protein expression data
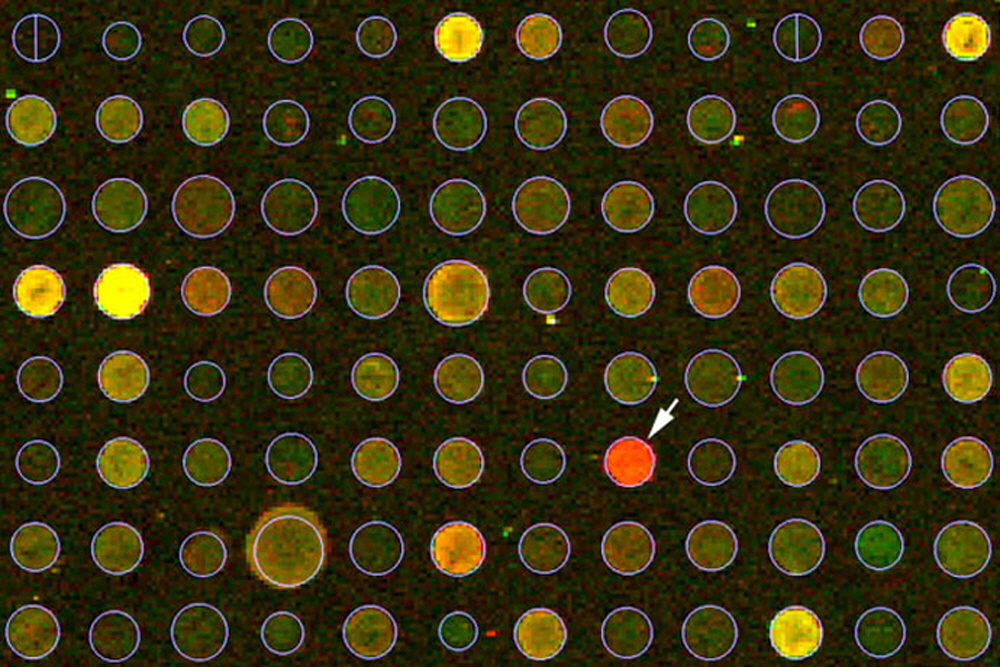
Figure 1. Arrayit Phase I Biomarker Discovery and Validation Services analysis of control and neurodegenerative disease samples. The biomarker lead (arrow) is easily identified in the H25K microarray scanned image.
Program Phases I-V
The five phases of our Biomarker and Therapeutic Protein Discovery and Validation program provide the deliverables described herein. Custom programs and value-added genotyping and protein expression information are also available.
Phase I (Biomarker leads):
- Pre-services conference
- Consulting, guidance and establishment of experimental design
- Assistance with sample preparation
- Assistance with RNA isolation procedures
- RNA analysis
- RNA amplification
- cDNA labeling
- cDNA analysis
- H25K hybridization, processing and scanning
- H25K data quantification
- Comparison of 1 control and 1 test sample
- Statistical analysis of 3.5 million data points
- Identification and up- and down-regulated genes
- Gene ontology analysis
- Genome Pathway Builder™ analysis
- Full report
- Post-analysis conference
Phase II (Biomarker scale-up):
- Pre-services conference
- Consulting, guidance and establishment of experimental design
- Assistance with sample preparation
- Assistance with RNA isolation procedures
- RNA analysis
- RNA amplification
- cDNA labeling
- cDNA analysis
- H25K hybridization, processing and scanning
- H25K data quantification
- Comparison of 10 control and 10 test samples
- Statistical analysis of 35 million data points
- Identification and up- and down-regulated genes
- Gene ontology analysis
- Genome Pathway Builder™ analysis
- Full report
- Post-analysis conference
Phase III (FDA data, 100 samples):
- Pre-services conference
- Consulting, guidance and establishment of experimental design
- Assistance with sample preparation
- Assistance with RNA isolation procedures
- RNA analysis
- RNA amplification
- cDNA labeling
- cDNA analysis
- H25K hybridization, processing and scanning
- H25K data quantification
- Comparison of 100 control and 100 test samples
- Statistical analysis of 350 million data points
- Identification and up- and down-regulated genes
- Gene ontology analysis
- Genome Pathway Builder™ analysis
- Full report
- Post-analysis conference
Phase IV (FDA data, 500 samples):
- Pre-services conference
- Consulting, guidance and establishment of experimental design
- Assistance with sample preparation
- Assistance with RNA isolation procedures
- RNA analysis
- RNA amplification
- cDNA labeling
- cDNA analysis
- H25K hybridization, processing and scanning
- H25K data quantification
- Comparison of 500 control and 500 test samples
- Statistical analysis of 1.75 billion data points
- Identification and up- and down-regulated genes
- Gene ontology analysis
- Genome Pathway Builder™ analysis
- Full report

