PCR Purification
Data Sheet
![]() Shop this product in our online store
Shop this product in our online store
Products - DNA Purification - PCR 384-Well 20 Kits to Purifiy 20 µl PCR Reactions for DNA Microarrays and DNA Sequencing Applications

Arrayit’s PCR Purification Kits allow high-throughput purification of PCR products for DNA microarrays, DNA sequencing, and other applications in genomics.
Table of Contents
- Introduction
- Quality Control
- Product Description
- Technical Notes
- Technical Assistance
- Short Protocol
- Complete Protocol
- Equipment and Reagents
- Troubleshooting Tips
- Kit Contents
- Scientific Publications
- Ordering Information
- Storage Conditions
- Warranty
Introduction
Congratulations on taking a big step towards improving the economies of scale, quality and speed of your genomics research. This booklet contains a complete set of protocols including the steps and principles needed to use Arrayit’s ArrayIt® brand 384-well PCR Purification Kit (PCR38420) for 20 µl PCR samples.
Quality Control
Arrayit assures the performance of these products. The finest scientific research went into the development of these products. Rigorous quality control monitoring on a lot-by-lot basis guarantees that the plates, buffers, and protocols conform to the highest industry standards.
Product Description
Arrayit’s ArrayIt® brand PCR Purification Kits are advanced purification systems that use sophisticated membrane separation technology in both 96-well and 384-well formats. PCR Purification Kits increase the quality of DNA microarray data by removing unwanted salts, enzymes, primers, unincorporated nucleotides, and other contaminants from amplified products that diminish the quality of DNA hybridization reactions and elevate fluorescent background.
Users will appreciate the following features:
- Improves spot signal intensities
- Reduces spot background
- Increases coupling efficiency
- Improves sample-to-sample printing consistency
- Supports native and modified PCR products
- Supports two- and three-dimensional microarray surfaces
- Superior SuperFilter separation chemistry provides >99% PCR purity
- >90% yield compared to 25-75% for other kits
- Purifies 50-10,000 bp PCR products
- Much better than ethanol precipitation
- No glass fiber shedding like other PCR Purification Kits
- Kits arrive ready to use, no buffer or column preparation required
- All buffers sterile-filtered for optimal performance
- Ultra-pure reagents used for superior performance
- Can be used manually or with automated liquid handling devices
- 96- and 384-well microplate formats provides high throughput
- Ideal for multi-patient genotyping applications
Technical Notes
Arrayit’s ArrayIt® 384-well 38420 PCR Purification Kit is designed for 20 µl PCR samples, but can also be used for smaller sample volumes ranging from 5-20 µl. For PCR samples smaller than 20 µl, simply scale down the volume of Binding Buffer proportionately. Samples can be eluted using 1X TE buffer (see Step 9), or distilled H2O in cases where Tris or EDTA may inhibit downstream reactions.
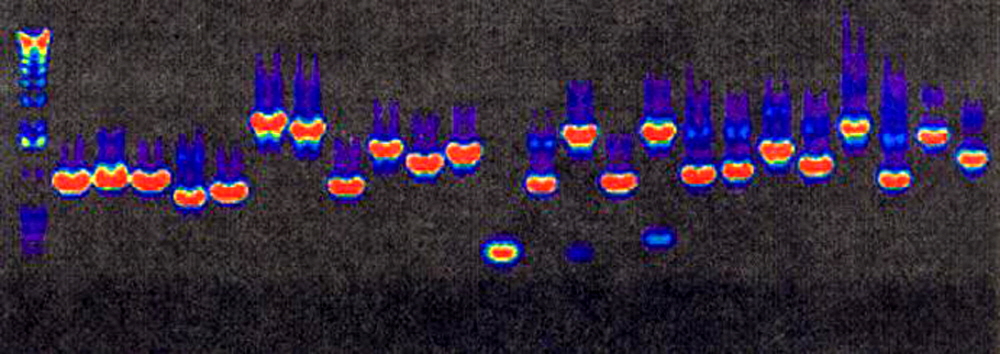
Figure 1. Gel electrophoresis products purified with Arrayit’s 96-well PCR Purification Kit (PCR96100). A 10 µl sample of each 100 µl reaction was separated on 0.8% agarose, stained with ethidium bromide, and the data were coded to a rainbow palette. Intense equimolar product bands are clearly visible. Similar results have been obtained with the PCR38420 Kit.
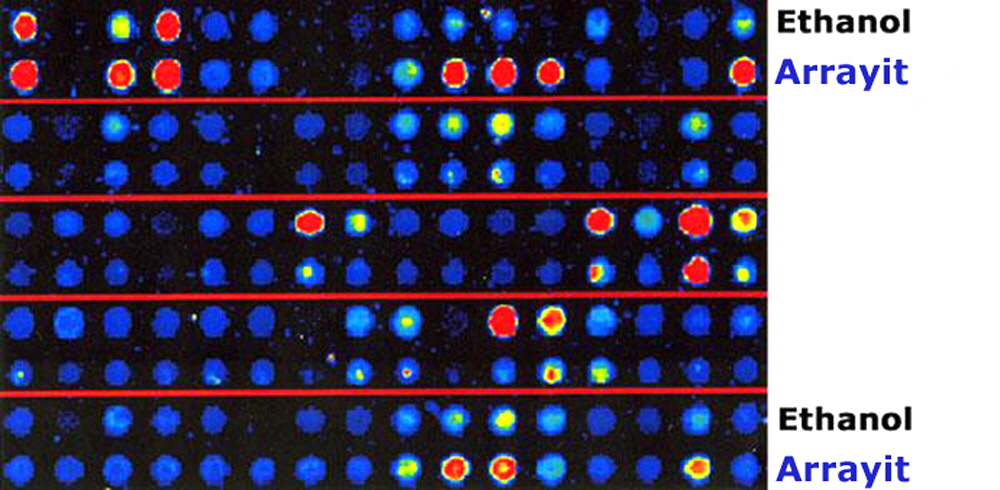
Figure 2. Microarray signal intensities generated with cDNA microarrays printed from PCR products purified by ethanol precipitation (Ethanol) or with Arrayit’s 96-well PCR Purification Kit (Arrayit). Microarrays were hybridized and scanned and the fluorescent data were coded to a rainbow palette. Stronger signals with PCR products purified with Arrayit’s PCR96100 Kit are easily observed, and similar results have been obtained with the PCR38420 Kit. Data provided courtesy of Hegde et al., BioTechniques 29, 548-550, 2000.
Technical Assistance
Please contact us if you have any comments, suggestions, or if you need technical assistance. By electronic email: arrayit@arrayit.com (under the subject heading please type ArrayIt technical assistance). Please remember that we want to hear about your successes!
Short Protocol (384-well for 20 µl PCR samples)
- Obtain 20 µl PCR samples.
- Add 4 volumes (80 µl) of 1.25X Binding Buffer to the SuperFilter100.
- Quickly add the 20 µl PCR samples to the SuperFilter100.
- Mix thoroughly by pipetting.
- Pass samples through SuperFilter100 by vacuum filtration.
- Wash 3 times with 100 µl of 1X Wash Buffer.
- Dry SuperFilter100 membrane completely by vacuum or centrifugation.
- Add 50 µl 0.1X TE (8.0) and incubate 5 min.
- Elute the purified PCR products into the 384-Well Marked Microplate.
- Dry the PCR products completely by vacuum centrifugation.
- Re-suspend in 2.5-5 µl Printing Solution.
- Print the purified PCR products onto Microarray Substrates.
Complete Protocol (384-well for 20 µl PCR samples)
- Obtain 20 µl PCR samples. Set up any polymerase chain reaction (PCR) of choice in a 20 µl volume. Standard PCR reactions contain 1X amplification buffer (50 mM KCl, 10 mM Tris-Cl pH 8.3, 1.5 mM MgCl2, 0.01% gelatin), 200 µM each of the four dNTPs, 20 pmoles each primer, 4-40 ng genomic DNA, and 0.8 unit of Taq DNA polymerase. A standard PCR cycling regime is 30 cycles at three temperatures (94°C, 50°C and 72°C), with 30 sec at each temperature. The PCR reactions can be run in a 384-well format and stored at 4°C or –20°C prior to purification.
- Add 4 volumes (80 µl) of 1.25X Binding Buffer to the SuperFilter100. Place the SuperFilter100 on a vacuum manifold and add 80 µl of 1.25X Binding Buffer to each well using an automatic pipette or liquid handling robot. This step should be performed within 60 sec to avoid loss of Binding Buffer, which passes slowly through the SuperFilter100 membrane by gravity flow.
- Quickly add the 20 µl PCR samples to the SuperFilter100. To the 80 µl of 1.25X Binding Buffer in each well, add the 20 µl PCR samples. Be careful not to splash the samples from well to well. This step can be performed manually by pipetting, or with an automated liquid handling device.
- Mix thoroughly by pipetting. Using a pipette or automated liquid handling device, mix the 80 µl of 1.25X Binding Buffer and 20 µl PCR samples by pipetting up and down at least 10 times. It is essential to mix the samples completely at this step, and failure to do so may reduce yield.
- Pass samples through SuperFilter100 by vacuum filtration. The mixed 100 µl samples should be passed slowly through the SuperFilter100 using a mild vacuum. The filtration process should take 30-60 seconds for efficient binding of the PCR products to the SuperFilter100 membrane. This step can also be performed by centrifugation for 60 sec at 500 x g. For the centrifugation protocol, fit the SuperFilter100 directly into the 384-Well Unmarked Microplate and spin for 60 sec at 500 x g. At the end of the centrifugation step, discard the eluent by inverting the 384-Well Unmarked Microplate.
- Wash 3 times with 100 µl of 1X Wash Buffer. After the 100 µl sample is filtered completely through the SuperFilter100, wash the SuperFilter100 with 3 times with 100 µl of 1X Wash Buffer. The wash steps will remove sample adhering to the walls of the SuperFilter100, as well as enzymes, salts, buffer components, nucleotides and primers from the SuperFilter membrane. Mild vacuum filtration or centrifugation for 60 sec at 500 x g into the 384-Well Unmarked Microplate can be used for the wash steps. For the centrifugation protocol, make certain to empty the 384-Well Unmarked Microplate after each centrifugation step. Do not allow unwashed samples to dry on the SuperFilter100 membrane prior to the wash steps, as this will lead to contamination of the PCR products.
- Dry SuperFilter100 membrane completely by vacuum or centrifugation. This step removes residual Wash Buffer remaining in the SuperFilter100 that can reduce yield. A 5 min vacuum treatment under strong vacuum or centrifugation for 5 min at 500 x g both work well for the drying step. For the centrifugation protocol, the 384-Well Unmarked Microplate should be used to catch the eluent and discarded after the drying step.
- Add 50 µl 0.1X TE (8.0) and incubate 5 min. Place the SuperFilter100 containing the bound PCR products onto a 384-Well Marked Microplate and add 50 µl of 0.1X TE pH 8.0 to each well. Pipette the 0.1X TE directly onto the membrane containing the bound PCR product. Do not use 1X TE as the EDTA in the concentrated sample may inhibit coupling to microarray substrates. After pipetting the 0.1X TE, wait 5 min before proceeding to the elution step. This step allows the bound PCR product to re-hydrate, and failure to wait 5 min prior to elution may reduce yield.
- Elute the purified PCR products into the 384-Well Marked Microplate. Centrifuge the two plates for 5 min at 500 x g to elute the purified PCR products into the 384-Well Marked Microplate. The volume of the eluted sample will be ~40 µl.
- Dry the PCR products completely by vacuum centrifugation. This step removes dH20 from the sample, leaving dry DNA pellets at the bottom of each well in the 384-Well Marked Microplate. A 30 minute vacuum centrifugation at 42-65°C should be sufficient to dry the samples completely, though additional drying time may be used without damaging the samples.
- Re-suspend in 2.5-5 µl Printing Solution. The dried and purified PCR products should be re-suspended in an appropriate printing solution such as Arrayit’s MicroSpotting Solution Plus. The yield from a typical 20 µl PCR sample will be 0.6-2.0 µg, producing a final DNA concentration of 0.12-0.5 µg/µl.
- Print the purified PCR products onto Microarray Substrates. High-quality microarray substrates such as SuperAmine, SuperAldehyde, SuperEpoxy, MirrorAmine, MirrorAldehyde, MirrorEpoxy should be used to obtain the best results. Users wishing to make their own substrates should use SuperClean or MirrorClean for best results. Optically flat substrates manufactured in a cleanroom environment consistently produce uniform spots, strong signal intensities and low and uniform background.
Equipment and Reagents
Vacuum Filter Manifold
Super Microarray Substrates
Mirror Substrates
Hybridization Solutions
Hybridization Cassettes
Cleanroom Wipes
Troubleshooting Tips
Purified PCR products contain contaminating primers
- May actually be primer dimers (small double-stranded products)
- Use less primer (≤20 pmoles per 20 µl reaction)
- Do not allow Binding Buffer dry on SuperFilter membrane
- Reduce Binding Buffer from 4 volumes to 3 volumes
Low yield
- Check efficiency of PCR reaction
- Poor elution due to residual Wash Buffer in the SuperFilter100
Well-to-well contamination
- Reduce Binding Buffer from 80 µl to 60 µl
- Use greater care when mixing Binding Buffer and PCR sample

Figure 3. 384-Well SuperFilter100 containing 384 samples of 80 µl of 1.25X Binding Buffer mixed with 20 µl PCR samples. The SuperFilter100 is positioned on a 384-Well Unmarked Microplate in preparation for the filtration step. Samples can be pipetted manually using a multi-channel pipetting device. The PCR38420 produces 90% yield and >99% sample purity. PCR purification is strongly recommended for all cDNA microarray applications.
Kit Contents (384-well for 20 µl PCR reactions)
- 1.25X Binding Buffer (35 ml)
- 1X Wash Buffer (150 ml)
- 384-Well SuperFilter100 (1)
- 384-Well Unmarked Microplate (1)
- 384-Well Marked Microplate and Cover (1)
- Handbook (1)
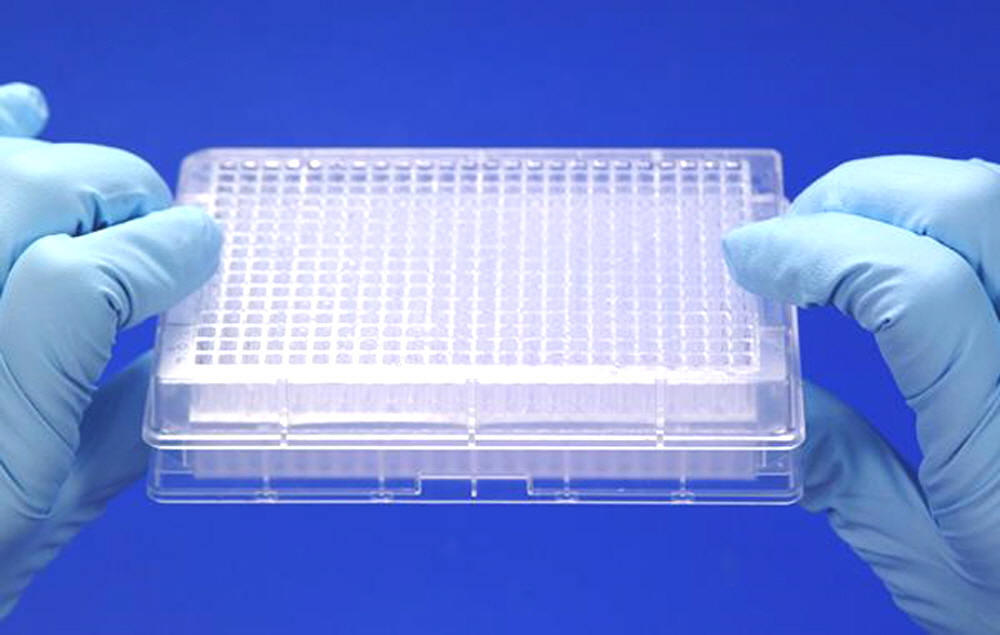
Figure 4. Shown is the SuperFilter100 containing 384 samples of 100 µl each, positioned onto a 384-well Unmarked Microplate. Sample filtration is performed by centrifugation for 5 min at 500 x g. The PCR38420 produces 90% yield and >99% sample purity. PCR purification is strongly recommended for all cDNA microarray applications.
Scientific Publications
Arrayit PCR Purification Kits are featured in hundreds of scientific publications.

